Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India
- PMID: 29217369
- PMCID: PMC5755003
- DOI: 10.1016/j.vaccine.2017.11.031
Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India
Abstract
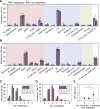
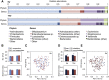
Oral rotavirus vaccines have consistently proven to be less immunogenic among infants in developing countries. Discrepancies in the intestinal microbiota, including a greater burden of enteropathogens and an altered commensal community composition, may contribute to this trend by inhibiting the replication of vaccine viruses. To test this possibility, we performed a nested case-control study in Vellore, India, in which we compared the intestinal microbiota of infants who responded serologically or not after two doses of Rotarix delivered at 6 and 10 weeks of age as part of a clinical trial (CTRI/2012/05/002677). The prevalence of 40 bacterial, viral, and eukaryotic pathogen targets was assessed in pre-vaccination stool samples from 325 infants using singleplex real-time PCR on a Taqman array card (TAC). In a subset of 170 infants, we assessed bacterial microbiota composition by sequencing the 16S rRNA gene V4 region. Contrary to expectations, responders were more likely than non-responders to harbor ≥1 bacterial enteropathogen at dose 1 (26% [40/156] vs 13% [21/157] of infants with TAC results who completed the study per protocol; χ2, P = .006), although this was not apparent at dose 2 (24% [38/158] vs 23% [36/158]; P = .790). Rotavirus shedding after dose 1 was negatively correlated with the replication of co-administered oral poliovirus vaccine (OPV). We observed no consistent differences in composition or diversity of the 16S bacterial microbiota according to serological response, although rotavirus shedding was associated with slightly more bacterial taxa pre-vaccination. Overall, our findings demonstrate an inhibitory effect of co-administered OPV on the first dose of Rotarix, consistent with previous studies, but in the context of OPV co-administration we did not find a strong association between other components of the intestinal microbiota at the time of vaccination and Rotarix immunogenicity.
Keywords: Enteropathogens; Immunogenicity; Microbiota; Rotarix; Rotavirus.
Copyright © 2017 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


Similar articles
-
Impact of maternal antibodies and microbiota development on the immunogenicity of oral rotavirus vaccine in African, Indian, and European infants.Nat Commun. 2021 Dec 15;12(1):7288. doi: 10.1038/s41467-021-27074-1. Nat Commun. 2021. PMID: 34911947 Free PMC article. Clinical Trial.
-
Non-interference of Bovine-Human reassortant pentavalent rotavirus vaccine ROTASIIL® with the immunogenicity of infant vaccines in comparison with a licensed rotavirus vaccine.Vaccine. 2018 Sep 5;36(37):5519-5523. doi: 10.1016/j.vaccine.2018.07.064. Epub 2018 Aug 10. Vaccine. 2018. PMID: 30104114 Free PMC article. Clinical Trial.
-
Breastfeeding linked to the reduction of both rotavirus shedding and IgA levels after Rotarix® immunization in Mexican infants.Vaccine. 2016 Oct 17;34(44):5284-5289. doi: 10.1016/j.vaccine.2016.09.006. Epub 2016 Sep 20. Vaccine. 2016. PMID: 27663670
-
Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines.Vaccine. 2012 Apr 27;30 Suppl 1:A30-5. doi: 10.1016/j.vaccine.2011.11.093. Vaccine. 2012. PMID: 22520134 Review.
-
Decreased performance of live attenuated, oral rotavirus vaccines in low-income settings: causes and contributing factors.Expert Rev Vaccines. 2018 Feb;17(2):145-161. doi: 10.1080/14760584.2018.1418665. Epub 2017 Dec 29. Expert Rev Vaccines. 2018. PMID: 29252042 Free PMC article. Review.
Cited by
-
Discovery of Predictors of Mycoplasma hyopneumoniae Vaccine Response Efficiency in Pigs: 16S rRNA Gene Fecal Microbiota Analysis.Microorganisms. 2020 Jul 29;8(8):1151. doi: 10.3390/microorganisms8081151. Microorganisms. 2020. PMID: 32751315 Free PMC article.
-
Diarrheal Etiology and Impact of Coinfections on Rotavirus Vaccine Efficacy Estimates in a Clinical Trial of a Monovalent Human-Bovine (116E) Oral Rotavirus Vaccine, Rotavac, India.Clin Infect Dis. 2019 Jul 2;69(2):243-250. doi: 10.1093/cid/ciy896. Clin Infect Dis. 2019. PMID: 30335135 Free PMC article. Clinical Trial.
-
Gut microbiota in vaccine naïve Gabonese children with rotavirus A gastroenteritis.Heliyon. 2024 Mar 30;10(7):e28727. doi: 10.1016/j.heliyon.2024.e28727. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38576575 Free PMC article.
-
Impact of maternal antibodies and microbiota development on the immunogenicity of oral rotavirus vaccine in African, Indian, and European infants.Nat Commun. 2021 Dec 15;12(1):7288. doi: 10.1038/s41467-021-27074-1. Nat Commun. 2021. PMID: 34911947 Free PMC article. Clinical Trial.
-
Bifidobacterium infantis supplementation versus placebo in early life to improve immunity in infants exposed to HIV: a protocol for a randomized trial.BMC Complement Med Ther. 2023 Oct 18;23(1):367. doi: 10.1186/s12906-023-04208-0. BMC Complement Med Ther. 2023. PMID: 37853370 Free PMC article.
References
-
- John J., Sarkar R., Muliyil J., Bhandari N., Bhan M.K., Kang G. Rotavirus gastroenteritis in India, 2011–2013: revised estimates of disease burden and potential impact of vaccines. Vaccine. 2014;32(Suppl. 1):A5–A9. - PubMed
-
- Madhi S.A., Cunliffe N.A., Steele D., Witte D., Kirsten M., Louw C. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Eng J Med. 2010;362:289–298. - PubMed
-
- Patriarca P.A., Wright P.F., John T.J. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–939. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

