Merkel Cell Carcinoma in the Age of Immunotherapy: Facts and Hopes
- PMID: 29217527
- PMCID: PMC5932211
- DOI: 10.1158/1078-0432.CCR-17-0439
Merkel Cell Carcinoma in the Age of Immunotherapy: Facts and Hopes
Abstract
Merkel cell carcinoma (MCC) is a rare (∼2,000 U.S. cases/year) but aggressive neuroendocrine tumor of the skin. For advanced MCC, cytotoxic chemotherapy only infrequently (<10% of cases) offers durable clinical responses (>1 year), suggesting a great need for improved therapeutic options. In 2008, the Merkel cell polyomavirus (MCPyV) was discovered and is clonally integrated in approximately 80% of MCC tumors. The remaining 20% of MCC tumors have large numbers of UV-associated mutations. Importantly, both the UV-induced neoantigens in virus-negative tumors and the MCPyV T antigen oncogenes that are required for virus-positive tumor growth are immunogenic. Indeed, antigen-specific T cells detected in patients are frequently dysfunctional/"exhausted," and the inhibitory ligand, PD-L1, is often present in MCC tumors. These findings led to recent clinical trials involving PD-1 pathway blockade in advanced MCC. The combined data from these trials involving three PD-1 pathway blocking agents-avelumab, pembrolizumab, and nivolumab-indicated a high frequency of durable responses in treated patients. Of note, prior treatment with chemotherapy was associated with decreased response rates to PD-1 checkpoint blockade. Over the past year, these striking data led to major changes in advanced MCC therapy, including the first-ever FDA drug approval for this disease. Despite these successes, approximately 50% of patients with MCC do not persistently benefit from PD-1 pathway blockade, underscoring the need for novel strategies to broaden antitumor immune responses in these patients. Here, we highlight recent progress in MCC including the underlying mechanisms of immune evasion and emerging approaches to augment the efficacy of PD-1 pathway blockade. Clin Cancer Res; 24(9); 2035-43. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest: Dr. Nghiem has received consulting fees from EMD Serono, Pfizer, and research grant support from Bristol-Meyers Squibb.
Figures

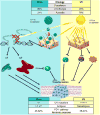
References
-
- Mattavelli I, Patuzzo R, Torri V, Gallino G, Maurichi A, Lamera M, et al. Prognostic factors in Merkel cell carcinoma patients undergoing sentinel node biopsy. Eur J Surg Oncol. 2017;43(8):1536–41. - PubMed
-
- Jouary T, Kubica E, Dalle S, Pages C, Duval-Modeste AB, Guillot B, et al. Sentinel node status and immunosuppression: recurrence factors in localized Merkel cell carcinoma. Acta Derm Venereol. 2015;95(7):835–40. - PubMed
-
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49(5):832–41. - PubMed
-
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89(1):1–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

