Aging and neurodegeneration are associated with increased mutations in single human neurons
- PMID: 29217584
- PMCID: PMC5831169
- DOI: 10.1126/science.aao4426
Aging and neurodegeneration are associated with increased mutations in single human neurons
Erratum in
-
Erratum for the Report "Aging and neurodegeneration are associated with increased mutations in single human neurons" by M. A. Lodato, R. E. Rodin, C. L. Bohrson, M. E. Coulter, A. R. Barton, M. Kwon, M. A. Sherman, C. M. Vitzthum, L. J. Luquette, C. N. Yandava, P. Yang, T. W. Chittenden, N. E. Hatem, S. C. Ryu, M. B. Woodworth, P. J. Park, C. A. Walsh.Science. 2018 Jul 6;361(6397):eaau6185. doi: 10.1126/science.aau6185. Science. 2018. PMID: 29976800 No abstract available.
Abstract
It has long been hypothesized that aging and neurodegeneration are associated with somatic mutation in neurons; however, methodological hurdles have prevented testing this hypothesis directly. We used single-cell whole-genome sequencing to perform genome-wide somatic single-nucleotide variant (sSNV) identification on DNA from 161 single neurons from the prefrontal cortex and hippocampus of 15 normal individuals (aged 4 months to 82 years), as well as 9 individuals affected by early-onset neurodegeneration due to genetic disorders of DNA repair (Cockayne syndrome and xeroderma pigmentosum). sSNVs increased approximately linearly with age in both areas (with a higher rate in hippocampus) and were more abundant in neurodegenerative disease. The accumulation of somatic mutations with age-which we term genosenium-shows age-related, region-related, and disease-related molecular signatures and may be important in other human age-associated conditions.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

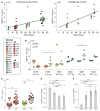

Comment in
-
Ageing: Somatic mutations accumulate in ageing and diseased neurons.Nat Rev Neurol. 2018 Feb;14(2):64. doi: 10.1038/nrneurol.2017.181. Epub 2017 Dec 22. Nat Rev Neurol. 2018. PMID: 29269785 No abstract available.
-
Tracing single-cell histories.Science. 2018 Feb 2;359(6375):521-522. doi: 10.1126/science.aar6335. Epub 2018 Feb 1. Science. 2018. PMID: 29420280 Free PMC article. No abstract available.
References
-
- Lu T, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. - PubMed
-
- Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JH. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol. 2014;15:465–481. - PubMed
-
- Giese H, Dolle ME, Hezel A, van Steeg H, Vijg J. Accelerated accumulation of somatic mutations in mice deficient in the nucleotide excision repair gene XPA. Oncogene. 1999;18:1257–1260. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

