Mapping the distribution of stem/progenitor cells across the mouse middle ear during homeostasis and inflammation
- PMID: 29217752
- PMCID: PMC5825877
- DOI: 10.1242/dev.154393
Mapping the distribution of stem/progenitor cells across the mouse middle ear during homeostasis and inflammation
Abstract
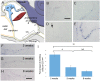
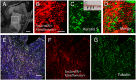
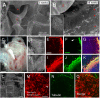
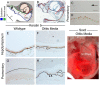
The middle ear epithelium is derived from neural crest and endoderm, which line distinct regions of the middle ear cavity. Here, we investigate the distribution of putative stem cell markers in the middle ear, combined with an analysis of the location of label-retaining cells (LRCs) to create a map of the middle ear mucosa. We show that proliferating cells and LRCs were associated with specific regions of the ear epithelium, concentrated in the hypotympanum at the base of the auditory bulla and around the ear drum. Sox2 was widely expressed in the endodermally derived ciliated pseudostratified epithelium of the hypotympanum. This part of the middle ear showed high levels of Wnt activity, as indicated by the expression of Axin2, a readout of Wnt signalling. Keratin 5 showed a more restricted expression within the basal cells of this region, with very little overlap between the Sox2- and keratin 5-positive epithelium, indicating that these genes mark distinct populations. Little expression of Sox2 or keratin 5 was observed in the neural crest-derived middle ear epithelium that lined the promontory, except in cases of otitis media when this epithelium underwent hyperplasia. This study lays the foundation for furthering our understanding of homeostasis and repair in the middle ear.
Keywords: Endoderm; Keratin 5; Label-retaining cells; Neural crest; Otitis media; Sox2.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Andoniadou C. L., Matsushima D., Mousavy Gharavy S. N., Signore M., Mackintosh A. I., Schaeffer M., Gaston-Massuet C., Mollard P., Jacques T. S., Le Tissier P. et al. (2013). Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 13, 433-445. 10.1016/j.stem.2013.07.004 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

