Opposing Roles of Dendritic Cell Subsets in Experimental GN
- PMID: 29217759
- PMCID: PMC5748909
- DOI: 10.1681/ASN.2017030270
Opposing Roles of Dendritic Cell Subsets in Experimental GN
Abstract
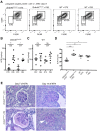
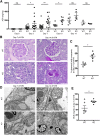
Dendritic cells (DCs) are thought to form a dendritic network across barrier surfaces and throughout organs, including the kidney, to perform an important sentinel function. However, previous studies of DC function used markers, such as CD11c or CX3CR1, that are not unique to DCs. Here, we evaluated the role of DCs in renal inflammation using a CD11c reporter mouse line and two mouse lines with DC-specific reporters, Zbtb46-GFP and Snx22-GFP. Multiphoton microscopy of kidney sections confirmed that most of the dendritically shaped CD11c+ cells forming a network throughout the renal interstitium expressed macrophage-specific markers. In contrast, DCs marked by Zbtb46-GFP or Snx22-GFP were less abundant, concentrated around blood vessels, and round in shape. We confirmed this pattern of localization using imaging mass cytometry. Motility measurements showed that resident macrophages were sessile, whereas DCs were motile before and after inflammation. Although uninflamed glomeruli rarely contained DCs, injury with nephrotoxic antibodies resulted in accumulation of ZBTB46 + cells in the periglomerular region. ZBTB46 identifies all classic DCs, which can be categorized into two functional subsets that express either CD103 or CD11b. Depletion of ZBTB46 + cells attenuated the antibody-induced kidney injury, whereas deficiency of the CD103+ subset accelerated injury through a mechanism that involved increased neutrophil infiltration. RNA sequencing 7 days after nephrotoxic antibody injection showed that CD11b+ DCs expressed the neutrophil-attracting cytokine CXCL2, whereas CD103+ DCs expressed high levels of several anti-inflammatory genes. These results provide new insights into the distinct functions of the two major DC subsets in glomerular inflammation.
Keywords: glomerular disease; glomerulonephritis; immunology.
Copyright © 2018 by the American Society of Nephrology.
Figures







Comment in
-
Renal Dendritic Cells: The Long and Winding Road.J Am Soc Nephrol. 2018 Jan;29(1):4-7. doi: 10.1681/ASN.2017101145. Epub 2017 Dec 11. J Am Soc Nephrol. 2018. PMID: 29229765 Free PMC article. No abstract available.
References
-
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker H-C: CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307: 254–258, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

