Autophagy in endometriosis
- PMID: 29218074
- PMCID: PMC5714760
Autophagy in endometriosis
Abstract
Endometriosis (EMS) is a common gynecologic disease that causes chronic pelvic pain, dysmenorrhea, and infertility in women. The doctrine of menstruation back flow planting and defects in the immune system are well known and widely accepted. In recent years, increasing studies have been focused on the role of autophagy in EMS, and have shown that autophagy plays a vital role in EMS. Autophagy, which is known as the non-apoptotic form of programmed cell death induced by a large number of intracellular/extracellular stimuli, is the major cellular pathway for the degradation of long-lived proteins and cytoplasmic organelles in eukaryotic cells. Autophagy commonly refers to macroautophagy, which is characterized by autophagosomes (double-membrane vesicles). In normal endometrial tissues, autophagy is induced in glandular epithelial and stromal cells throughout the menstrual cycle. However, aberrant autophagy occurs in the eutopic endometrium and ectopic endometriotic foci, which contributes to the pathogenesis of EMS by promoting the hyperplasia of endometriotic tissues and stromal cells, restricting apoptosis, and inducing abnormal immune responses. Consistent with changes in autophagy levels between normal endometria, eutopic and ectopic endometria from patients with EMS, the altered expression of autophagy-related genes (ATGs) is also observed. Currently, many factors are involved in the aberrant autophagy of endometriotic tissues, including female hormones, certain drugs, hypoxia, and oxidative stress. Therefore, studies focusing on autophagy may uncover a new potential treatment for EMS. The aim of this review is to discuss the role of aberrant autophagy in EMS and to explore the potential value of autophagy as a target for EMS therapy.
Keywords: Autophagy; autophagy-related genes; endometrial stromal cells; endometriosis.
Conflict of interest statement
None.
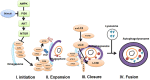
Figures


References
-
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. - PubMed
-
- Pritts EA, Taylor RN. An evidence-based evaluation of endometriosis-associated infertility. Endocrinol Metab Clin North Am. 2003;32:653–667. - PubMed
-
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. - PubMed
-
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. - PubMed
-
- Frackiewicz EJ. Endometriosis: an overview of the disease and its treatment. J Am Pharm Assoc (Wash) 2000;40:645–657. quiz 699-702. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
