14-3-3 proteins: an important regulator of autophagy in diseases
- PMID: 29218076
- PMCID: PMC5714762
14-3-3 proteins: an important regulator of autophagy in diseases
Abstract
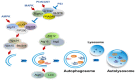
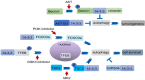
Autophagy is a cell digestion process that determines cell fate by promoting cell survival or inducing cell death in a cell context-dependent manner. Several classical signaling pathways, such as phosphoinositide-3-kinase and mammalian target of rapamycin, tightly regulate autophagy. 14-3-3 proteins regulate various signaling pathways by phosphorylation-dependent binding with partner proteins. 14-3-3 proteins also regulate autophagy by binding with autophagy-related proteins such as Beclin-1 and hVPS34. This review summarizes the role of 14-3-3 proteins in the control of autophagy in cancer, neurodegenerative diseases and other pathological conditions.
Keywords: 14-3-3 proteins; autophagy; cancer; neurodegenerative diseases.
Conflict of interest statement
None.
Figures

References
-
- Levine B, Klionsky DJ. Development by selfdigestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. - PubMed
-
- Mortimore GE, Lardeux BR, Adams CE. Regulation of microautophagy and basal protein turnover in rat liver. Effects of short-term starvation. J Biol Chem. 1988;263:2506–2512. - PubMed
-
- Schmitt R, Melk A. Molecular mechanisms of renal aging. Kidney Int. 2017;92:569–579. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
