Hsp90 inhibitor sensitizes TRAIL-mediated apoptosis via chop-dependent DR5 upregulation in colon cancer cells
- PMID: 29218092
- PMCID: PMC5714778
Hsp90 inhibitor sensitizes TRAIL-mediated apoptosis via chop-dependent DR5 upregulation in colon cancer cells
Abstract
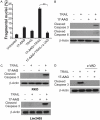
Heat shock protein 90 (Hsp90), a molecular chaperone, is involved in a variety of physiological and pathological processes. Targeting Hsp90 by small molecules has been developed as an attractive strategy of anticancer therapy. In this study, we investigated the mechanism of Hsp90 inhibitor suppresses CRC growth and potentiates effects of other chemotherapeutic drugs. We found that Hsp90 inhibitor induces chop-dependent DR5 upregulation regardless of p53 status. Furthermore, DR5 is required for Hsp90 inhibitor-induced apoptosis. Hsp90 inhibitor also synergized with TRAIL to induce marked apoptosis via DR5 in CRC. Overall, our results illustrate DR5 play a key role in mediating the anticancer effects of Hsp90 inhibitor in CRC and suggest that DR5 expression can be used as an indicator of Hsp90 inhibitor sensitivity, which has important implications for it clinical applications.
Keywords: DR5; Hsp90 inhibitor; TRAIL; apoptosis; chop.
Conflict of interest statement
None.
Figures





Similar articles
-
Ginsenoside compound K sensitizes human colon cancer cells to TRAIL-induced apoptosis via autophagy-dependent and -independent DR5 upregulation.Cell Death Dis. 2016 Aug 11;7(8):e2334. doi: 10.1038/cddis.2016.234. Cell Death Dis. 2016. PMID: 27512955 Free PMC article.
-
Ixazomib promotes CHOP-dependent DR5 induction and apoptosis in colorectal cancer cells.Cancer Biol Ther. 2019;20(3):284-294. doi: 10.1080/15384047.2018.1529095. Epub 2018 Oct 25. Cancer Biol Ther. 2019. PMID: 30359552 Free PMC article.
-
Hsp90 Inhibitor SNX-2112 Enhances TRAIL-Induced Apoptosis of Human Cervical Cancer Cells via the ROS-Mediated JNK-p53-Autophagy-DR5 Pathway.Oxid Med Cell Longev. 2019 Mar 25;2019:9675450. doi: 10.1155/2019/9675450. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31019655 Free PMC article.
-
Dasatinib promotes TRAIL-mediated apoptosis by upregulating CHOP-dependent death receptor 5 in gastric cancer.FEBS Open Bio. 2018 Mar 23;8(5):732-742. doi: 10.1002/2211-5463.12404. eCollection 2018 May. FEBS Open Bio. 2018. PMID: 29744288 Free PMC article.
-
Advances in the study of death receptor 5.Front Pharmacol. 2025 Mar 12;16:1549808. doi: 10.3389/fphar.2025.1549808. eCollection 2025. Front Pharmacol. 2025. PMID: 40144653 Free PMC article. Review.
Cited by
-
Death Receptor 5 (TNFRSF10B) Is Upregulated and TRAIL Resistance Is Reversed in Hypoxia and Normoxia in Colorectal Cancer Cell Lines after Treatment with Skyrin, the Active Metabolite of Hypericum spp.Cancers (Basel). 2021 Apr 1;13(7):1646. doi: 10.3390/cancers13071646. Cancers (Basel). 2021. PMID: 33916015 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Goldberg RM. Therapy for metastatic colorectal cancer. Oncologist. 2006;11:981–987. - PubMed
-
- Chua CS, Low H, Sim TS. Co-chaperones of Hsp90 in Plasmodium falciparum and their concerted roles in cellular regulation. Parasitology. 2014;141:1177–1191. - PubMed
-
- Sidera K, Patsavoudi E. HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat Anticancer Drug Discov. 2014;9:1–20. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
