Scutellarin suppresses human colorectal cancer metastasis and angiogenesis by targeting ephrinb2
- PMID: 29218107
- PMCID: PMC5714793
Scutellarin suppresses human colorectal cancer metastasis and angiogenesis by targeting ephrinb2
Abstract
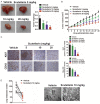
Tumor induced angiogenesis is an attractive target for anti-cancer drug treatment. Scutellarin, which is a native compound derived from scutellaria altissima leaves, has already been proved to possess anti-tumor activities. Nevertheless, their effects in colorectal cancer metastasis and angiogenesis have not been evaluated. In order to reveal the anti-angiogenic and anti-metastasis capacity of scutellarin, wound healing and Transwell chamber inserts invasion were done in colorectal cancer cells, and cell proliferation as wells colony formation were conducted to identify the proliferation inhibition of colorectal cancer in vitro. The growth inhibition of scutellarin was further definite by a mouse colorectal xenograft model in vivo. Herein, we demonstrated scutellarin suppressed colorectal cancer cell viability and colony formation in vitro, and remarkably reduced tumor growth in vivo mouse xenografts. Additionally, scutellarin restrained colorectal cancer cells-induced angiogenesis, inhibited human umbilical vascular endothelial cells (HUVECs) migration, tube formation of HUVECs, and micro-vessel formation in chick embnyo chorioallantoic menbreme (CAM) assay. Altogether, our results exhibited the evidence that scutellarin inhibit colorectal cancer angiogenesis and metastasis via targeting ephrinb2 signaling, with the potential of an anti-tumor agent for cancer treatment.
Keywords: Colorectal cancer; angiogenesis; ephrinb2; metastasis; scutellarin.
Conflict of interest statement
None.
Figures



References
-
- Huang Y, Duan H, Sun Y. Elevated expression of SAG/ROC2/Rbx2/Hrt2 in human colon carcinomas: SAG does not induce neoplastic transformation, but antisense SAG transfection inhibits tumor cell growth. Mol Carcinog. 2001;30:62–70. - PubMed
-
- Chan JY, Tan BK, Lee SC. Scutellarin sensitizes drug-evoked colon cancer cell apoptosis through enhanced caspase-6 activation. Anticancer Res. 2009;29:3043–3047. - PubMed
LinkOut - more resources
Full Text Sources
