Downregulation of cathepsin G reduces the activation of CD4+ T cells in murine autoimmune diabetes
- PMID: 29218110
- PMCID: PMC5714796
Downregulation of cathepsin G reduces the activation of CD4+ T cells in murine autoimmune diabetes
Abstract
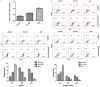
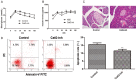
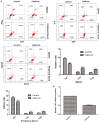
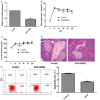
Type 1 diabetes mellitus (T1DM) is an autoimmune disease due to progressive injury of islet cells mediated by T lymphocytes (T cells). Our previous studies have shown that only cathepsin G (CatG), not other proteases, is involved in the antigen presentation of proinsulin, and if the presentation is inhibited, the activation of CD4+ T cells induced by proinsulin is alleviated in T1DM patients, and CatG-specific inhibitor reduces the activation of CD4+ cells induced by proinsulin in T1DM patients. Therefore, we hypothesize that CatG may play an important role in the activation of CD4+ T cells in T1DM. To this end, mouse studies were conducted to demonstrate that CatG impacts the activation of CD4+ T cells in non-obese diabetic (NOD) mice. CatG gene expression and the activation of CD4+ T cells were examined in NOD mice. The effect of CatG inhibitor was investigated in NOD mice on the activation of CD4+ T cells, islet β cell function, islet inflammation and β-cell apoptosis. Furthermore, NOD mice were injected with CatG siRNA in early stage to observe the effect of CatG knockdown on the activation status of CD4+ T cells and the progression of diabetes. During the pathogenesis of diabetes, the expression level of CatG in NOD mice gradually increased and the CD4+ T cells were gradually activated, resulting in more TH1 cells and less TH2 and Treg cells. Treatment with CatG-specific inhibitor reduced the blood glucose level, improved the function of islet β cells and reduced the activation of CD4+ T cells. Early application of CatG siRNA improved the function of islet β cells, reduced islet inflammation and β cell apoptosis, and lowered the activation level of CD4+ T cells, thus slowing down the progression of diabetes.
Keywords: CD4+ T cells; NOD mice; cathepsin G; type 1 diabetes mellitus.
Conflict of interest statement
None.
Figures
References
-
- Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. - PubMed
-
- Roep BO, Tree TI. Immune modulation in humans: implications for type 1 diabetes mellitus. Nat Rev Endocrinol. 2014;10:229–242. - PubMed
-
- Lernmark A, Larsson HE. Immune therapy in type 1 diabetes mellitus. Nat Rev Endocrinol. 2013;9:92–103. - PubMed
-
- Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, Sanda S, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS Type 1 Diabetes TrialNet Canakinumab Study Group; Pickersgill L, de Koning E, Ziegler AG, Boehm B, Badenhoop K, Schloot N, Bak JF, Pozzilli P, Mauricio D, Donath MY, Castano L, Wagner A, Lervang HH, Perrild H, Mandrup-Poulsen T AIDA Study Group. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–1915. - PMC - PubMed
-
- Ludvigsson J, Krisky D, Casas R, Battelino T, Castano L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ, Veeze HJ, Palmer J, Samuelsson U, Elding Larsson H, Aman J, Kardell G, Neiderud Helsingborg J, Lundstrom G, Albinsson E, Carlsson A, Nordvall M, Fors H, Arvidsson CG, Edvardson S, Hanas R, Larsson K, Rathsman B, Forsgren H, Desaix H, Forsander G, Nilsson NO, Akesson CG, Keskinen P, Veijola R, Talvitie T, Raile K, Kapellen T, Burger W, Neu A, Engelsberger I, Heidtmann B, Bechtold S, Leslie D, Chiarelli F, Cicognani A, Chiumello G, Cerutti F, Zuccotti GV, Gomez Gila A, Rica I, Barrio R, Clemente M, Lopez Garcia MJ, Rodriguez M, Gonzalez I, Lopez JP, Oyarzabal M, Reeser HM, Nuboer R, Stouthart P, Bratina N, Bratanic N, de Kerdanet M, Weill J, Ser N, Barat P, Bertrand AM, Carel JC, Reynaud R, Coutant R, Baron S. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433–442. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
