Basis and Statistical Design of the Passive HIV-1 Antibody Mediated Prevention (AMP) Test-of-Concept Efficacy Trials
- PMID: 29218117
- PMCID: PMC5714515
- DOI: 10.1515/scid-2016-0001
Basis and Statistical Design of the Passive HIV-1 Antibody Mediated Prevention (AMP) Test-of-Concept Efficacy Trials
Abstract
Background: Anti-HIV-1 broadly neutralizing antibodies (bnAbs) have been developed as potential agents for prevention of HIV-1 infection. The HIV Vaccine Trials Network and the HIV Prevention Trials Network are conducting the Antibody Mediated Prevention (AMP) trials to assess whether, and how, intravenous infusion of the anti-CD4 binding site bnAb, VRC01, prevents HIV-1 infection. These are the first test-of-concept studies to assess HIV-1 bnAb prevention efficacy in humans.
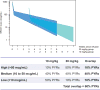
Methods: The AMP trials are two parallel phase 2b HIV-1 prevention efficacy trials conducted in two cohorts: 2700 HIV-uninfected men and transgender persons who have sex with men in the United States, Peru, Brazil, and Switzerland; and 1500 HIV-uninfected sexually active women in seven countries in sub-Saharan Africa. Participants are randomized 1:1:1 to receive an intravenous infusion of 10 mg/kg VRC01, 30 mg/kg VRC01, or a control preparation every 8 weeks for a total of 10 infusions. Each trial is designed (1) to assess overall prevention efficacy (PE) pooled over the two VRC01 dose groups vs. control and (2) to assess VRC01 dose and laboratory markers as correlates of protection (CoPs) against overall and genotype- and phenotype-specific infection.
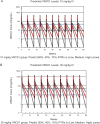
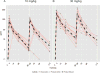
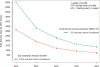
Results: Each AMP trial is designed to have 90% power to detect PE > 0% if PE is ≥ 60%. The AMP trials are also designed to identify VRC01 properties (i.e., concentration and effector functions) that correlate with protection and to provide insight into mechanistic CoPs. CoPs are assessed using data from breakthrough HIV-1 infections, including genetic sequences and sensitivities to VRC01-mediated neutralization and Fc effector functions.
Conclusions: The AMP trials test whether VRC01 can prevent HIV-1 infection in two study populations. If affirmative, they will provide information for estimating the optimal dosage of VRC01 (or subsequent derivatives) and identify threshold levels of neutralization and Fc effector functions associated with high-level protection, setting a benchmark for future vaccine evaluation and constituting a bridge to other bnAb approaches for HIV-1 prevention.
Keywords: Clinical trial; Correlates of protection; HIV prevention; Monoclonal antibody; Sieve analysis; Surrogate endpoint; VRC01.
Conflict of interest statement
Conflict of interest statement: All authors have no potential conflicts of interest to declare.
Figures


References
-
- AIDSinfo. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents: Limitations to Treatment Safety and Efficacy: Adverse Effects of Antiretroviral Agents. 2014 Last Modified 5/1/2014 Accessed 2/17/2015. http://aidsinfo.nih.gov/guidelines.
-
- Asmal M, Sun Y, Lane S, et al. Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses. Journal of Virology. 2011;85(11):5465–5475. - PMC - PubMed
-
- Baba TW, Liska V, Hofmann-Lehmann R, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature Medicine. 2000;6(2):200–206. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
