Insights on spin polarization through the spin density source function
- PMID: 29218155
- PMCID: PMC5707457
- DOI: 10.1039/c4sc03988b
Insights on spin polarization through the spin density source function
Abstract
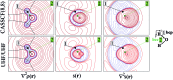
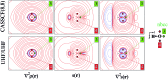
Understanding how spin information is transmitted from paramagnetic to non-magnetic centers is crucial in advanced materials research and calls for novel interpretive tools. Herein, we show that the spin density at a point may be seen as determined by a local source function for such density, operating at all other points of space. Integration of the local source over Bader's quantum atoms measures their contribution in determining the spin polarization at any system's location. Each contribution may be then conveniently decomposed in a magnetic term due to the magnetic natural orbital(s) density and in a reaction or relaxation term due to the remaining orbitals density. A simple test case, 3B1 water, is chosen to exemplify whether an atom or group of atoms concur or oppose the paramagnetic center in determining a given local spin polarization. Discriminating magnetic from reaction or relaxation contributions to such behaviour strongly enhances chemical insight, though care needs to be paid to the large sensitivity of the latter contributions to the level of the computational approach and to the difficulty of singling out the magnetic orbitals in the case of highly correlated systems. Comparison of source function atomic contributions to the spin density with those reconstructing the electron density at a system's position, enlightens how the mechanisms which determine the two densities may in general differ and how diverse may be the role played by each system's atom in determining each of the two densities. These mechanisms reflect the quite diverse portraits of the electron density and electron spin density Laplacians, hence the different local concentration/dilution of the total and (α-β) electron densities throughout the system. Being defined in terms of an observable, the source function for the spin density is also potentially amenable to experimental determination, as customarily performed for its electron density analogue.
Figures

References
-
- Zhang Q., Li B., Chen L. Inorg. Chem. 2013;52:9356–9362. - PubMed
-
- Eisenträger A., Vella D., Griffiths I. M. Appl. Phys. Lett. 2014;105:033508.
-
- Ohno H., Chiba D., Matsukura F., Omiya T., Abe E., Dietl T., Ohno Y., Ohtani K. Nature. 2000;408:944–946. - PubMed
-
- Coey J. M. D., Magnetism and Magnetic Materials, Cambridge University Press, Cambridge, U.K., 2010, ISBN 13: 9780521816144.
LinkOut - more resources
Full Text Sources
Other Literature Sources

