Rim-Differentiated C5-Symmetric Tiara-Pillar[5]arenes
- PMID: 29220153
- PMCID: PMC5765533
- DOI: 10.1021/jacs.7b10767
Rim-Differentiated C5-Symmetric Tiara-Pillar[5]arenes
Abstract
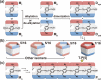
The synthesis of "rim-differentiated" C5-symmetric pillar[5]arenes, whose two rims are decorated with different chemical functionalities, has remained a challenging task. This is due to the inherent statistical nature of the cyclization of 1,4-disubstituted alkoxybenzenes with different substituents, which leads to four constitutional isomers with only 1/16th being rim-differentiated. Herein, we report a "preoriented" synthetic protocol based on FeCl3-catalyzed cyclization of asymmetrically substituted 2,5-dialkoxybenzyl alcohols. This yields an unprecedented 55% selectivity of the C5-symmetric tiara-like pillar[5]arene isomer among four constitutional isomers. Based on this new method, a series of functionalizable tiara-pillar[5]arene derivatives with C5-symmetry was successfully synthesized, isolated, and fully characterized in the solid state.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

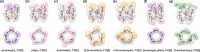
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

