N-Hydroxyphthalimide-Mediated Electrochemical Iodination of Methylarenes and Comparison to Electron-Transfer-Initiated C-H Functionalization
- PMID: 29220181
- PMCID: PMC5831155
- DOI: 10.1021/jacs.7b09744
N-Hydroxyphthalimide-Mediated Electrochemical Iodination of Methylarenes and Comparison to Electron-Transfer-Initiated C-H Functionalization
Abstract
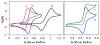
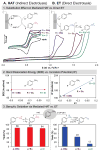
An electrochemical method has been developed for selective benzylic iodination of methylarenes. The reactions feature the first use of N-hydroxyphthalimide as an electrochemical mediator for C-H oxidation to nonoxygenated products. The method provides the basis for direct (in situ) or sequential benzylation of diverse nucleophiles using methylarenes as the alkylating agent. The hydrogen-atom transfer mechanism for C-H iodination allows C-H oxidation to proceed with minimal dependence on the substrate electronic properties and at electrode potentials 0.5-1.2 V lower than that of direct electrochemical C-H oxidation.
Figures

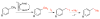

References
-
- Shono T, editor. Electroorganic Synthesis. Academic Press; London: 1991.
- Little RD, Weinberg NL, editors. Electroorganic Synthesis. Marcel Dekker; New York: 1991.
- Grimshaw J. Electrochemical Reactions and Mechanisms in Organic Chemistry. Elsevier; Amsterdam: 2000.
- Hammerich O, Speiser B, editors. Organic Electrochemistry. 5. CRC Press; Boca Raton: 2015.
-
-
For recent reviews, see: Moeller KD. Tetrahedron. 2000;56:9527.Sperry JB, Wright DL. Chem Soc Rev. 2006;35:605.Yoshida JI, Kataoka K, Horcajada R, Nagaki A. Chem Rev. 2008;108:2265.Horn EJ, Rosen BR, Baran PS. ACS Cent Sci. 2016;2:302.
-
-
- Steckhan E. Angew Chem, Int Ed. 1986;28:683.
- Ogibin YN, Elinson MN, Nikishin GI. Russ Chem Rev. 2009;78:89.
- Francke R, Little RD. Chem Soc Rev. 2014;43:2492. - PubMed
-
-
For leading references, see: Masui M, Hara S, Ueshima T, Kawaguchi T, Ozaki S. Chem Pharm Bull. 1983;31:4209.Masui M, Kawaguchi T, Ozaki S. J Chem Soc, Chem Commun. 1985:1484.
-
-
-
For reviews, see: Ishii Y, Sakaguchi S, Iwahama T. Adv Synth Catal. 2001;343:393.Ishii Y, Sakaguchi S. J Synth Org Chem, Jpn. 2003;61:1056.
-
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

