Substitutions of PrP N-terminal histidine residues modulate scrapie disease pathogenesis and incubation time in transgenic mice
- PMID: 29220360
- PMCID: PMC5722314
- DOI: 10.1371/journal.pone.0188989
Substitutions of PrP N-terminal histidine residues modulate scrapie disease pathogenesis and incubation time in transgenic mice
Abstract
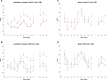
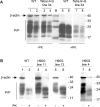
Prion diseases have been linked to impaired copper homeostasis and copper induced-oxidative damage to the brain. Divalent metal ions, such as Cu2+ and Zn2+, bind to cellular prion protein (PrPC) at octapeptide repeat (OR) and non-OR sites within the N-terminal half of the protein but information on the impact of such binding on conversion to the misfolded isoform often derives from studies using either OR and non-OR peptides or bacterially-expressed recombinant PrP. Here we created new transgenic mouse lines expressing PrP with disrupted copper binding sites within all four histidine-containing OR's (sites 1-4, H60G, H68G, H76G, H84G, "TetraH>G" allele) or at site 5 (composed of residues His-95 and His-110; "H95G" allele) and monitored the formation of misfolded PrP in vivo. Novel transgenic mice expressing PrP(TetraH>G) at levels comparable to wild-type (wt) controls were susceptible to mouse-adapted scrapie strain RML but showed significantly prolonged incubation times. In contrast, amino acid replacement at residue 95 accelerated disease progression in corresponding PrP(H95G) mice. Neuropathological lesions in terminally ill transgenic mice were similar to scrapie-infected wt controls, but less severe. The pattern of PrPSc deposition, however, was not synaptic as seen in wt animals, but instead dense globular plaque-like accumulations of PrPSc in TgPrP(TetraH>G) mice and diffuse PrPSc deposition in (TgPrP(H95G) mice), were observed throughout all brain sections. We conclude that OR and site 5 histidine substitutions have divergent phenotypic impacts and that cis interactions between the OR region and the site 5 region modulate pathogenic outcomes by affecting the PrP globular domain.
Conflict of interest statement
Figures




Similar articles
-
Strain-dependent role of copper in prion disease through binding to histidine residues in the N-terminal domain of prion protein.J Neurochem. 2023 Nov;167(3):394-409. doi: 10.1111/jnc.15971. Epub 2023 Sep 30. J Neurochem. 2023. PMID: 37777338
-
Prion Protein Devoid of the Octapeptide Repeat Region Delays Bovine Spongiform Encephalopathy Pathogenesis in Mice.J Virol. 2017 Dec 14;92(1):e01368-17. doi: 10.1128/JVI.01368-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046443 Free PMC article.
-
Re-transmissibility of mouse-adapted ME7 scrapie strain to ovine PrP transgenic mice.J Vet Sci. 2019 Mar;20(2):e8. doi: 10.4142/jvs.2019.20.e8. Epub 2019 Mar 12. J Vet Sci. 2019. PMID: 30944531 Free PMC article.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
-
Novel mechanisms of degeneration of the central nervous system--prion structure and biology.Ciba Found Symp. 1988;135:239-60. doi: 10.1002/9780470513613.ch16. Ciba Found Symp. 1988. PMID: 2900720 Review.
Cited by
-
Structural Consequences of Copper Binding to the Prion Protein.Cells. 2019 Jul 25;8(8):770. doi: 10.3390/cells8080770. Cells. 2019. PMID: 31349611 Free PMC article. Review.
-
Strain-Dependent Prion Infection in Mice Expressing Prion Protein with Deletion of Central Residues 91-106.Int J Mol Sci. 2020 Oct 1;21(19):7260. doi: 10.3390/ijms21197260. Int J Mol Sci. 2020. PMID: 33019549 Free PMC article.
-
Deciphering Copper Coordination in the Mammalian Prion Protein Amyloidogenic Domain.Biophys J. 2020 Feb 4;118(3):676-687. doi: 10.1016/j.bpj.2019.12.025. Epub 2020 Jan 3. Biophys J. 2020. PMID: 31952810 Free PMC article.
-
Mutations in Prion Protein Gene: Pathogenic Mechanisms in C-Terminal vs. N-Terminal Domain, a Review.Int J Mol Sci. 2019 Jul 23;20(14):3606. doi: 10.3390/ijms20143606. Int J Mol Sci. 2019. PMID: 31340582 Free PMC article. Review.
-
Copper coordination modulates prion conversion and infectivity in mammalian prion proteins.Prion. 2023 Dec;17(1):1-6. doi: 10.1080/19336896.2022.2163835. Prion. 2023. PMID: 36597284 Free PMC article. Review.
References
-
- Prusiner SB. Prions. Proc Natl Acad Sci 1998; 95: 13363–83. doi: 10.1073/pnas.95.23.13363 - DOI - PMC - PubMed
-
- Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, Wuthrich K. NMR structure of the mouse prion protein domain PrP (121–231). Nature 1996; 382: 180–3. doi: 10.1038/382180a0 - DOI - PubMed
-
- Horiuchi M, Caughey B. Specific binding of normal prion protein to the scrapie form via a localized domain initiates its conversion to the protease-resistant state. Embo J. 1999; 18: 3193–203. doi: 10.1093/emboj/18.12.3193 - DOI - PMC - PubMed
-
- Kocisko DA, Come JH, Priola SA, Chesebro B, Raymond GJ, Lansbury PT Jr., et al. Cell-free formation of protease-resistant prion protein. Nature 1994; 370: 471–4. doi: 10.1038/370471a0 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

