The effectiveness and limitation of the national childhood hepatitis A vaccination program in the Republic of Korea: Findings from the Korean National Health and Nutrition Examination Survey (KNHANES), 2015
- PMID: 29220416
- PMCID: PMC5722338
- DOI: 10.1371/journal.pone.0189210
The effectiveness and limitation of the national childhood hepatitis A vaccination program in the Republic of Korea: Findings from the Korean National Health and Nutrition Examination Survey (KNHANES), 2015
Abstract
Background: Vaccination for hepatitis A virus (HAV) has been implemented as one of the national vaccination programs despite the epidemiological transition of HAV in the Republic of Korea. While the national HAV vaccination program is largely associated with the shift of socioeconomic trend in the country, concerns have been raised on the effectiveness of the HAV immunization. The objective of this study was to examine the epidemiological trend of HAV and assess the effectiveness of the nationwide HAV vaccination policy based on a nationally representative sample of the Korean population collected in 2015.
Methods: We analyzed anti-HAV of 5,856 respondents aged ≥10 years collected from Korean National Health and Nutrition Examination Survey (KNHANES) data in 2015. We estimated age-adjusted anti-HAV prevalence by sociodemographic and other characteristics. We evaluated the factors associated with anti-HAV positivity among each age group (10-19, 20-29, 30-45 and over 45 years old).
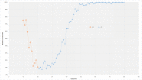
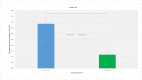
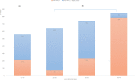
Results: The prevalence of anti-HAV among adults aged ≥10 years was 72.5% (95% confidence interval, CI, 73.7-71.4) in 2015. The lowest age-specific prevalence was among adults aged 20-29 years with 11.9% (95% CI 9.3-15.1%). The prevalence of anti-HAV among those aged 10-14 and 15-19 years was 59.7% (95% CI 52.7-66.4) and 24.0% (95% CI 19.5-29.3), respectively. The prevalence of anti-HAV among adults aged between 30 and 44 years rapidly increased from below 20% to above 90%. The prevalence of anti-HAV among adults aged ≥45 years was 97.8% (95% CI 96.0-97.6). Factors significantly associated with anti-HAV positivity among those aged 10-19 years old were young age, higher house income and high influenza vaccination rate. Compared to the respondents aged 10-19 years (those who were subject to the national childhood vaccine recommendation), those aged 20-29 years (those who were not subject to the recommendation) had low adjusted odds ratio (OR, 0.52 95% CI 0.34-.81 P-value = 0.004) for anti-HAV positivity.
Conclusions: The age-adjusted anti-HAV prevalence showed a U-shaped association, implying the high dependence of anti-HAV prevalence on age and the epidemiological shift. The inclusion of the hepatitis A vaccine into the national immunization recommendation was effective shown by the increase of immunity in the general population. However, the vaccination rate was low in the low-income group. Young adults aged 20-39 years may benefit from inclusion in the HAV vaccination program due to the significantly low vaccination rate.
Conflict of interest statement
Figures
Similar articles
-
Decreasing immunity to hepatitis A virus infection among US adults: Findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012.Vaccine. 2015 Nov 17;33(46):6192-8. doi: 10.1016/j.vaccine.2015.10.009. Epub 2015 Oct 23. Vaccine. 2015. PMID: 26476364
-
[Prevalence of hepatitis A antibody among population covered by different hepatitis A immunization strategies in Shandong Province, 2015, China].Zhonghua Yu Fang Yi Xue Za Zhi. 2017 Jun 6;51(6):480-483. doi: 10.3760/cma.j.issn.0253-9624.2017.06.005. Zhonghua Yu Fang Yi Xue Za Zhi. 2017. PMID: 28592089 Chinese.
-
Self-reported hepatitis A vaccination as a predictor of hepatitis A virus antibody protection in U.S. adults: National Health and Nutrition Examination Survey 2007-2012.Vaccine. 2015 Jul 31;33(32):3887-93. doi: 10.1016/j.vaccine.2015.06.063. Epub 2015 Jun 24. Vaccine. 2015. PMID: 26116252 Free PMC article.
-
Increasing incidence of hepatitis A in Korean adults.Intervirology. 2010;53(1):10-4. doi: 10.1159/000252778. Epub 2010 Jan 5. Intervirology. 2010. PMID: 20068335 Review.
-
[Current status and vaccine indication for hepatitis A virus infection in Korea].Korean J Gastroenterol. 2008 Jun;51(6):331-7. Korean J Gastroenterol. 2008. PMID: 18604134 Review. Korean.
Cited by
-
The Shifting Epidemiology of Hepatitis A in the World Health Organization Western Pacific Region.Vaccines (Basel). 2024 Feb 16;12(2):204. doi: 10.3390/vaccines12020204. Vaccines (Basel). 2024. PMID: 38400187 Free PMC article. Review.
-
Gender differences in hepatitis A seropositivity rates according to the Republic of Korea's vaccination policy.Osong Public Health Res Perspect. 2024 Apr;15(2):168-173. doi: 10.24171/j.phrp.2023.0263. Epub 2024 Apr 16. Osong Public Health Res Perspect. 2024. PMID: 38621763 Free PMC article.
-
Causes and countermeasures for repeated outbreaks of hepatitis A among adults in Korea.Epidemiol Health. 2019;41:e2019038. doi: 10.4178/epih.e2019038. Epub 2019 Sep 22. Epidemiol Health. 2019. PMID: 31715685 Free PMC article.
References
-
- Siegl G, Frosner GG, Gauss-Muller V, Tratschin JD, Deinhardt F. The physicochemical properties of infectious hepatitis A virions. The Journal of general virology. 1981;57(Pt 2):331–41. Epub 1981/12/01. doi: 10.1099/0022-1317-57-2-331 . - DOI - PubMed
-
- Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006;43(2 Suppl 1):S164–72. Epub 2006/02/01. doi: 10.1002/hep.21052 . - DOI - PubMed
-
- Brundage SC, Fitzpatrick AN. Hepatitis A. American family physician. 2006;73(12):2162–8. Epub 2006/07/20. . - PubMed
-
- Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015;12(12):e1001923 Epub 2015/12/04. doi: 10.1371/journal.pmed.1001923 ; PubMed Central PMCID: PMCPMC4668832. - DOI - PMC - PubMed
-
- Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–7. Epub 2010/08/21. doi: 10.1016/j.vaccine.2010.08.037 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

