Brain Responses to Cigarette-Related and Emotional Images in Smokers During Smoking Cessation: No Effect of Varenicline or Bupropion on the Late Positive Potential
- PMID: 29220524
- PMCID: PMC6329398
- DOI: 10.1093/ntr/ntx264
Brain Responses to Cigarette-Related and Emotional Images in Smokers During Smoking Cessation: No Effect of Varenicline or Bupropion on the Late Positive Potential
Abstract
Introduction: Varenicline and bupropion are two effective smoking cessation pharmacotherapies. Researchers have hypothesized that they might be effective, in part, because they reduce cue reactivity and cue-induced cravings. Here, we used event-related potentials (ERPs) to directly measure brain responses to cigarette-related and other motivationally relevant images during a pharmacologically aided quit attempt.
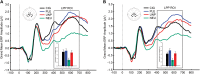
Methods: Smokers involved in a 12-week placebo-controlled double-blind clinical trial of smoking cessation medications (varenicline, bupropion, placebo) took part in the study. We assessed participants at two time points: 24 h (n = 140) and 4 weeks (n = 176) after the quit date. At both sessions, we measured the amplitude of the late positive potential (LPP), an ERP component reliably associated with motivational relevance, and self-reported tonic craving using the brief version of the Questionnaire of Smoking Urges (QSU-Brief).
Results: At both sessions, emotional and cigarette-related images evoked significantly larger LPPs than neutral images. Neither drug type nor smoking abstinence altered this effect at either session. At both sessions, varenicline and bupropion significantly reduced self-reported tonic craving relative to the placebo condition.
Conclusions: While both varenicline and bupropion reduced self-reported tonic craving, neither medication altered the amplitude of the LPP to cigarette-related or emotional pictures in smokers attempting to quit. These medications may influence abstinence by means other than by reducing neuroaffective responses to cigarette-related cues. Smokers should be prepared for the likelihood that even after several weeks of successful abstinence, once treatment ends, cigarette-related cues may remain motivationally relevant and trigger cravings that might lead to relapse.
Implications: Bupropion and varenicline do not alter electrophysiological responses, as measured by the LPP, to cigarette-related and emotional images. These findings help explain why cigarette-related cues can trigger relapse when smoking cessation medication treatments end.
Figures
References
-
- Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. - PubMed
-
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):346–347.
-
- Tønnesen P, Tonstad S, Hjalmarson A, et al. . A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. J Intern Med. 2003;254(2):184–192. - PubMed
-
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. 2004;72(4):712–722. - PubMed
-
- Gonzales D, Rennard SI, Nides M, et al. ; Varenicline Phase 3 Study Group Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

