Multi-omic Mitoprotease Profiling Defines a Role for Oct1p in Coenzyme Q Production
- PMID: 29220658
- PMCID: PMC5730362
- DOI: 10.1016/j.molcel.2017.11.023
Multi-omic Mitoprotease Profiling Defines a Role for Oct1p in Coenzyme Q Production
Abstract
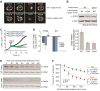
Mitoproteases are becoming recognized as key regulators of diverse mitochondrial functions, although their direct substrates are often difficult to discern. Through multi-omic profiling of diverse Saccharomyces cerevisiae mitoprotease deletion strains, we predicted numerous associations between mitoproteases and distinct mitochondrial processes. These include a strong association between the mitochondrial matrix octapeptidase Oct1p and coenzyme Q (CoQ) biosynthesis-a pathway essential for mitochondrial respiration. Through Edman sequencing and in vitro and in vivo biochemistry, we demonstrated that Oct1p directly processes the N terminus of the CoQ-related methyltransferase, Coq5p, which markedly improves its stability. A single mutation to the Oct1p recognition motif in Coq5p disrupted its processing in vivo, leading to CoQ deficiency and respiratory incompetence. This work defines the Oct1p processing of Coq5p as an essential post-translational event for proper CoQ production. Additionally, our data visualization tool enables efficient exploration of mitoprotease profiles that can serve as the basis for future mechanistic investigations.
Keywords: Coq5p; MIPEP; Oct1p; coenzyme Q; mitochondria; mitoproteases; multi-omic; oligopeptidase; protease; transomic; ubiquinone.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



References
-
- Ashby MN, Kutsunai SY, Ackerman S, Tzagoloff A, Edwards PA. COQ2 is a candidate for the structural gene encoding para-hydroxybenzoate:polyprenyltransferase. J Biol Chem. 1992;267:4128–4136. - PubMed
-
- Avci D, Fuchs S, Schrul B, Fukumori A, Breker M, Frumkin I, Chen CY, Biniossek ML, Kremmer E, Schilling O, et al. The yeast ER-intramembrane protease Ypf1 refines nutrient sensing by regulating transporter abundance. Mol Cell. 2014;56:630–640. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

