A tRNA fragment, tRF5-Glu, regulates BCAR3 expression and proliferation in ovarian cancer cells
- PMID: 29221134
- PMCID: PMC5707028
- DOI: 10.18632/oncotarget.20709
A tRNA fragment, tRF5-Glu, regulates BCAR3 expression and proliferation in ovarian cancer cells
Abstract
Ovarian cancer is a complex disease marked by tumor heterogeneity, which contributes to difficulties in diagnosis and treatment. New molecular targets and better molecular profiles defining subsets of patients are needed. tRNA fragments (tRFs) offer a recently identified group of noncoding RNAs that are often as abundant as microRNAs in cancer cells. Initially their presence in deep sequencing data sets was attributed to the breakdown of mature tRNAs, however, it is now clear that they are actively generated and function in multiple regulatory events. One such tRF, a 5' fragment of tRNA-Glu-CTC (tRF5-Glu), is processed from the mature tRNA-Glu and is shown in this study to be expressed in ovarian cancer cells. We confirmed that tRF5-Glu binds directly to a site in the 3'UTR of the Breast Cancer Anti-Estrogen Resistance 3 (BCAR3) mRNA thereby down regulating its expression. BCAR3 has not previously been studied in ovarian cancer cells and our studies demonstrate that inhibiting BCAR3 expression suppresses ovarian cancer cell proliferation. Furthermore, mimics of tRF5-Glu were found to inhibit proliferation of ovarian cancer cells. In summary, BCAR3 and tRF5-Glu contribute to the complex tumor heterogeneity of ovarian cancer cells and may provide new targets for therapeutic intervention.
Keywords: BCAR3; noncoding RNA; ovarian cancer; tRF5-Glu; tRNA fragments.
Conflict of interest statement
CONFLICTS OF INTEREST The authors state no conflicts of interest.
Figures
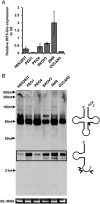
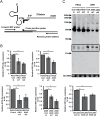
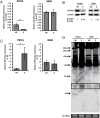
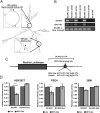


References
-
- National Academies of Sciences E, Medicine . Ovarian Cancers: Evolving Paradigms in Research and Care. National Academies Press; Washington, DC: 2016. - PubMed
-
- Levine DA, Karlan BY, Strauss JF. Evolving Approaches in Research and Care for Ovarian Cancers: A Report From the National Academies of Sciences, Engineering, and Medicine. JAMA. 2016;315:1943–4. - PubMed
-
- Konecny GE, Winterhoff B, Wang C. Gene-expression signatures in ovarian cancer: Promise and challenges for patient stratification. Gynecologic oncology. 2016;141:379–85. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

