Hepatitis B virus X protein inhibits apoptosis by modulating endoplasmic reticulum stress response
- PMID: 29221184
- PMCID: PMC5707078
- DOI: 10.18632/oncotarget.21630
Hepatitis B virus X protein inhibits apoptosis by modulating endoplasmic reticulum stress response
Abstract
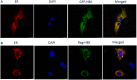
Chronic Hepatitis B virus (HBV) infection is a major risk of hepatocellular carcinoma (HCC) worldwide. Hepatitis B virus X protein (HBx) is encoded by one of the four open reading frames of HBV, and is well known as an important coactivator for HBV replication and HBV-associated hepatocellular carcinogenesis. However, its role in keeping cells from apoptosis to promote HCC proliferation remains controversial. Here, we used HBx expressing HCC cells as a model, to investigate the mechanism of HBx-mediated cellular response to endoplasmic reticulum (ER) stress. We found that HBx protein was localized in ER lumen and interacted with GRP78 directly. This interaction resulted in suppression of eIF2α phosphorylation, inhibited expression of ATF4/CHOP/Bcl-2, and reduced cleavage of poly ADP-ribose polymerase (PARP) and level of γH2AX, thus preventing HCC cells from cell death and negatively regulating DNA repair. This study reveals a novel mechanism of the HBx-mediated oncogenesis and provides a basis for potential HBx-targeted therapeutic intervention of HCC.
Keywords: ER stress; HBV; HBx; apoptosis; hepatocellular carcinoma.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no competing interests.
Figures




References
-
- Huang JL, Ren TY, Cao SW, Zheng SH, Hu XM, Hu YW, Lin L, Chen J, Zheng L, Wang Q. HBx-related long non-coding RNA DBH-AS1 promotes cell proliferation and survival by activating MAPK signaling in hepatocellular carcinoma. Oncotarget. 2015;6:33791–33804. https://doi.org/10.18632/oncotarget.5667. - DOI - PMC - PubMed
-
- Yu Z, Gao YQ, Feng H, Lee YY, Li MS, Tian Y, Go MY, Yu DY, Cheung YS, Lai PB, Yu J, Wong VW, Sung JJ, et al. Cell cycle-related kinase mediates viral-host signaling to promote hepatitis B virus-associated hepatocarcinogenesis. Gut. 2014;63:1793–1804. https://doi.org/10.1136/gutjnl-2013-305584. - DOI - PubMed
-
- Liu B, Fang M, He Z, Cui D, Jia S, Lin X, Xu X, Zhou T, Liu W. Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf2 activation. Cell Death Dis. 2015;6:e1980. https://doi.org/10.1038/cddis.2015.322. - DOI - PMC - PubMed
-
- Liu B, Fang M, Hu Y, Huang B, Li N, Chang C, Huang R, Xu X, Yang Z, Chen Z, Liu W. Hepatitis B virus X protein inhibits autophagic degradation by impairing lysosomal maturation. Autophagy. 2014;10:416–430. https://doi.org/10.4161/auto.27286. - DOI - PMC - PubMed
-
- Zhu R, Mok MT, Kang W, Lau SS, Yip WK, Chen Y, Lai PB, Wong VW, To KF, Sung JJ, Cheng AS, Chan HL. Truncated HBx-dependent silencing of GAS2 promotes hepatocarcinogenesis through deregulation of cell cycle, senescence and p53-mediated apoptosis. J Pathol. 2015;237:38–49. https://doi.org/10.1002/path.4554. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

