Direct HPV E6/Myc interactions induce histone modifications, Pol II phosphorylation, and hTERT promoter activation
- PMID: 29221209
- PMCID: PMC5707103
- DOI: 10.18632/oncotarget.22036
Direct HPV E6/Myc interactions induce histone modifications, Pol II phosphorylation, and hTERT promoter activation
Abstract
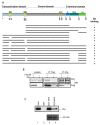
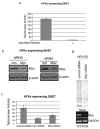
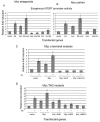
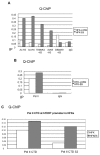
Human Papillomavirus Viruses (HPVs) are associated with the majority of human cervical and anal cancers and 10-30% of head and neck squamous carcinomas. E6 oncoprotein from high risk HPVs interacts with the p53 tumor suppressor protein to facilitate its degradation and increases telomerase activity for extending the life span of host cells. We published previously that the Myc cellular transcription factor associates with the high-risk HPV E6 protein in vivo and participates in the transactivation of the hTERT promoter. In the present study, we further analyzed the role of E6 and the Myc-Max-Mad network in regulating the hTERT promoter. We confirmed that E6 and Myc interact independently and that Max can also form a complex with E6. However, the E6/Max complex is observed only in the presence of Myc, suggesting that E6 associates with Myc/Max dimers. Consistent with the hypothesis that Myc is required for E6 induction of the hTERT promoter, Myc antagonists (Mad or Mnt) significantly blocked E6-mediated transactivation of the hTERT promoter. Analysis of Myc mutants demonstrated that both the transactivation domain and HLH domain of Myc protein were required for binding E6 and for the consequent transactivation of the hTERT promoter, by either Myc or E6. We also showed that E6 increased phosphorylation of Pol II on the hTERT promoter and induced epigenetic histone modifications of the hTERT promoter. More important, knockdown of Myc expression dramatically decreased engagement of acetyl-histones and Pol II at the hTERT promoter in E6-expressing cells. Thus, E6/Myc interaction triggers the transactivation of the hTERT promoter by modulating both histone modifications, Pol II phosphorylation and promoter engagement, suggesting a novel mechanism for telomerase activation and a new target for HPV- associated human cancer.
Keywords: RNA polymerase II; histones; oncoproteins; papillomaviruses; telomerase.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no competing interests.
Figures


References
-
- Fu B, Quintero J, Baker CC. Keratinocyte growth conditions modulate telomerase expression, senescence, and immortalization by human papillomavirus type 16 E6 and E7 oncogenes. Cancer Res. 2003;63:7815–24. - PubMed
-
- Gewin L, Galloway DA. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J Virol. 2001;75:7198–201. https://doi.org/10.1128/JVI.75.15.7198-7201.2001. - DOI - PMC - PubMed
-
- Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. https://doi.org/10.1038/380079a0. - DOI - PubMed
-
- Liu X, Yuan H, Fu B, Disbrow GL, Apolinario T, Tomaic V, Kelley ML, Baker CC, Huibregtse J, Schlegel R. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J Biol Chem. 2005;280:10807–16. https://doi.org/10.1074/jbc.M410343200. - DOI - PubMed
-
- Oh ST, Kyo S, Laimins LA. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J Virol. 2001;75:5559–66. https://doi.org/10.1128/JVI.75.12.5559-5566.2001. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

