Evolutionary characterization and transcript profiling of β-tubulin genes in flax (Linum usitatissimum L.) during plant development
- PMID: 29221437
- PMCID: PMC5721616
- DOI: 10.1186/s12870-017-1186-0
Evolutionary characterization and transcript profiling of β-tubulin genes in flax (Linum usitatissimum L.) during plant development
Abstract
Background: Microtubules, polymerized from alpha and beta-tubulin monomers, play a fundamental role in plant morphogenesis, determining the cell division plane, the direction of cell expansion and the deposition of cell wall material. During polarized pollen tube elongation, microtubules serve as tracks for vesicular transport and deposition of proteins/lipids at the tip membrane. Such functions are controlled by cortical microtubule arrays. Aim of this study was to first characterize the flax β-tubulin family by sequence and phylogenetic analysis and to investigate differential expression of β-tubulin genes possibly related to fibre elongation and to flower development.
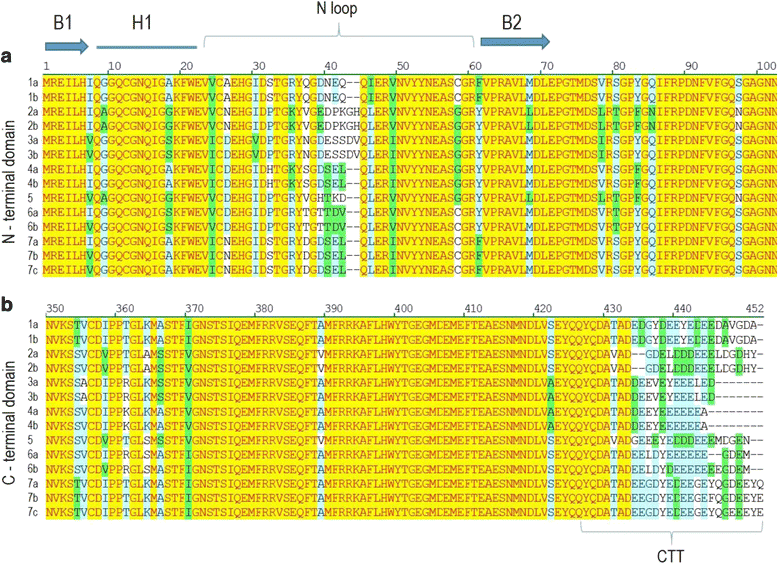
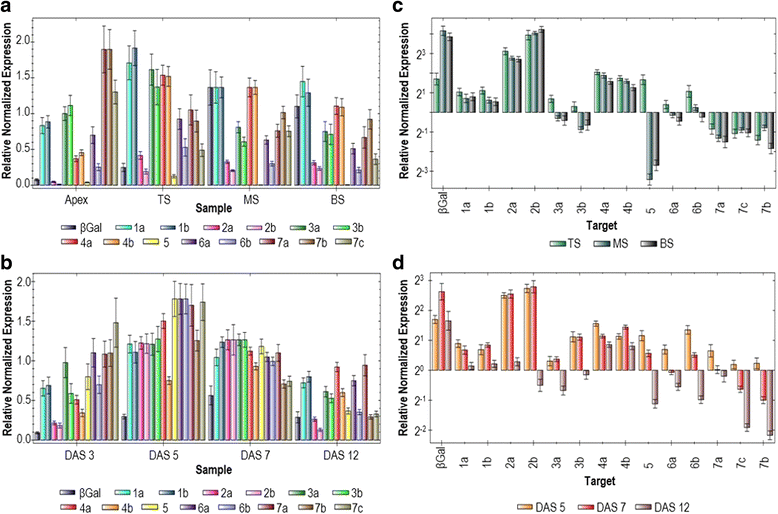
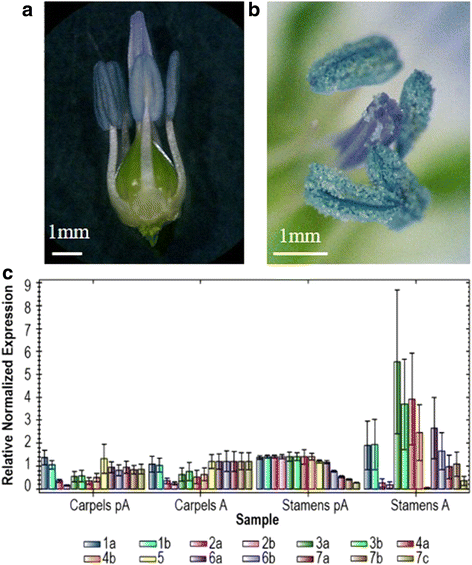
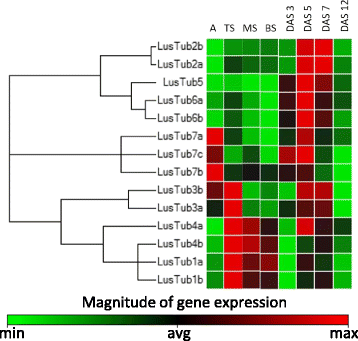
Results: We report the cloning and characterization of the complete flax β-tubulin gene family: exon-intron organization, duplicated gene comparison, phylogenetic analysis and expression pattern during stem and hypocotyl elongation and during flower development. Sequence analysis of the fourteen expressed β-tubulin genes revealed that the recent whole genome duplication of the flax genome was followed by massive retention of duplicated tubulin genes. Expression analysis showed that β-tubulin mRNA profiles gradually changed along with phloem fibre development in both the stem and hypocotyl. In flowers, changes in relative tubulin transcript levels took place at anthesis in anthers, but not in carpels.
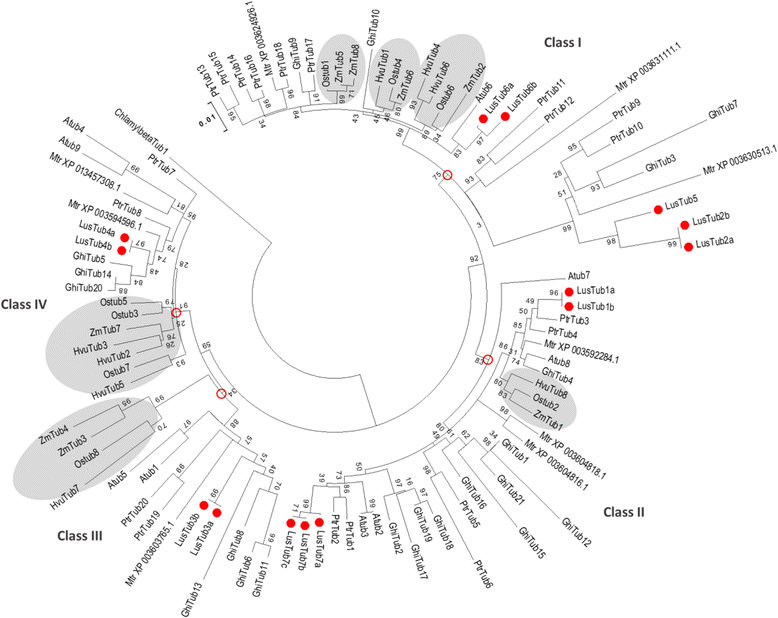
Conclusions: Phylogenetic analysis supports the origin of extant plant β-tubulin genes from four ancestral genes pre-dating angiosperm separation. Expression analysis suggests that particular tubulin subpopulations are more suitable to sustain different microtubule functions such as cell elongation, cell wall thickening or pollen tube growth. Tubulin genes possibly related to different microtubule functions were identified as candidate for more detailed studies.
Keywords: Bast fibres; Duplicated genes; Expression analysis; Flax; Gene family; Phylogenetic analysis; Pollen tube; β-tubulin.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Flax tubulin and CesA superfamilies represent attractive and challenging targets for a variety of genome- and base-editing applications.Funct Integr Genomics. 2020 Jan;20(1):163-176. doi: 10.1007/s10142-019-00667-2. Epub 2019 Mar 2. Funct Integr Genomics. 2020. PMID: 30826923 Review.
-
Microarray analysis of developing flax hypocotyls identifies novel transcripts correlated with specific stages of phloem fibre differentiation.Ann Bot. 2008 Sep;102(3):317-30. doi: 10.1093/aob/mcn110. Epub 2008 Jun 30. Ann Bot. 2008. PMID: 18593690 Free PMC article.
-
Microarray analysis of flax (Linum usitatissimum L.) stems identifies transcripts enriched in fibre-bearing phloem tissues.Mol Genet Genomics. 2007 Aug;278(2):149-65. doi: 10.1007/s00438-007-0241-1. Epub 2007 May 15. Mol Genet Genomics. 2007. PMID: 17503083
-
Genome-wide identification, phylogenetic classification, and exon-intron structure characterization of the tubulin and actin genes in flax (Linum usitatissimum).Cell Biol Int. 2019 Sep;43(9):1010-1019. doi: 10.1002/cbin.11001. Epub 2018 Jul 8. Cell Biol Int. 2019. PMID: 29885094
-
Genome-wide identification of ATP binding cassette (ABC) transporter and heavy metal associated (HMA) gene families in flax (Linum usitatissimum L.).BMC Genomics. 2020 Oct 19;21(1):722. doi: 10.1186/s12864-020-07121-9. BMC Genomics. 2020. PMID: 33076828 Free PMC article. Review.
Cited by
-
Genome-Wide Analysis of Tubulin Gene Family in Cassava and Expression of Family Member FtsZ2-1 during Various Stress.Plants (Basel). 2021 Mar 31;10(4):668. doi: 10.3390/plants10040668. Plants (Basel). 2021. PMID: 33807152 Free PMC article.
-
Tubulin-Based DNA Barcode: Principle and Applications to Complex Food Matrices.Genes (Basel). 2019 Mar 18;10(3):229. doi: 10.3390/genes10030229. Genes (Basel). 2019. PMID: 30889932 Free PMC article. Review.
-
Flax tubulin and CesA superfamilies represent attractive and challenging targets for a variety of genome- and base-editing applications.Funct Integr Genomics. 2020 Jan;20(1):163-176. doi: 10.1007/s10142-019-00667-2. Epub 2019 Mar 2. Funct Integr Genomics. 2020. PMID: 30826923 Review.
-
Microscopic and Transcriptomic Analysis of Pollination Processes in Self-Incompatible Taraxacum koksaghyz.Plants (Basel). 2021 Mar 16;10(3):555. doi: 10.3390/plants10030555. Plants (Basel). 2021. PMID: 33809548 Free PMC article.
-
MiRNAs differentially expressed in vegetative and reproductive organs of Marchantia polymorpha - insights into their expression pattern, gene structures and function.RNA Biol. 2024 Jan;21(1):1-12. doi: 10.1080/15476286.2024.2303555. Epub 2024 Feb 1. RNA Biol. 2024. PMID: 38303117 Free PMC article.
References
-
- Gorshkova T, Sal'nikova V, Chemikosova S, Ageeva M, Pavlencheva N, van Dam J. The snap point: a transition point in Linum usitatissimum bast fiber development. Ind Crop Prod. 2003;18(3):213–221. doi: 10.1016/S0926-6690(03)00043-8. - DOI
-
- Gorshkova T, Gurjanov O, Mikshina P, Ibragimova N, Mokshina N, Salnikov V, Ageeva M, Amenitskii S, Chernova T, Chemikosova S. Specific type of secondary cell wall formed by plant fibers. Russ J Plant Physiol. 2010;57(3):328–341. doi: 10.1134/S1021443710030040. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

