A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease
- PMID: 29221491
- PMCID: PMC5723032
- DOI: 10.1186/s13195-017-0318-y
A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease
Erratum in
-
Correction to: A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease.Alzheimers Res Ther. 2018 Sep 27;10(1):99. doi: 10.1186/s13195-018-0409-4. Alzheimers Res Ther. 2018. PMID: 30261916 Free PMC article.
Abstract
Background: Gantenerumab is a fully human monoclonal antibody that binds aggregated amyloid-β (Aβ) and removes Aβ plaques by Fc receptor-mediated phagocytosis. In the SCarlet RoAD trial, we assessed the efficacy and safety of gantenerumab in prodromal Alzheimer's disease (AD).
Methods: In this randomized, double-blind, placebo-controlled phase III study, we investigated gantenerumab over 2 years. Patients were randomized to gantenerumab 105 mg or 225 mg or placebo every 4 weeks by subcutaneous injection. The primary endpoint was the change from baseline to week 104 in Clinical Dementia Rating Sum of Boxes (CDR-SB) score. We evaluated treatment effects on cerebrospinal fluid biomarkers (all patients) and amyloid positron emission tomography (substudy). A futility analysis was performed once 50% of patients completed 2 years of treatment. Safety was assessed in patients who received at least one dose.
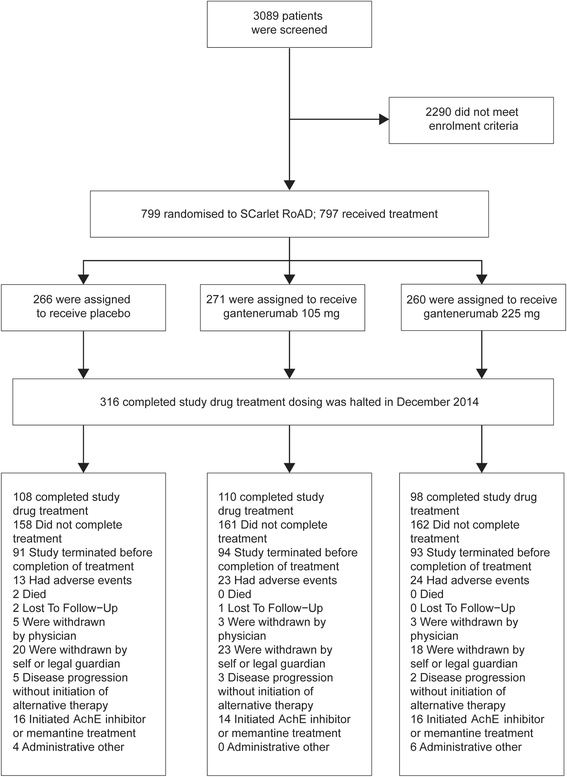
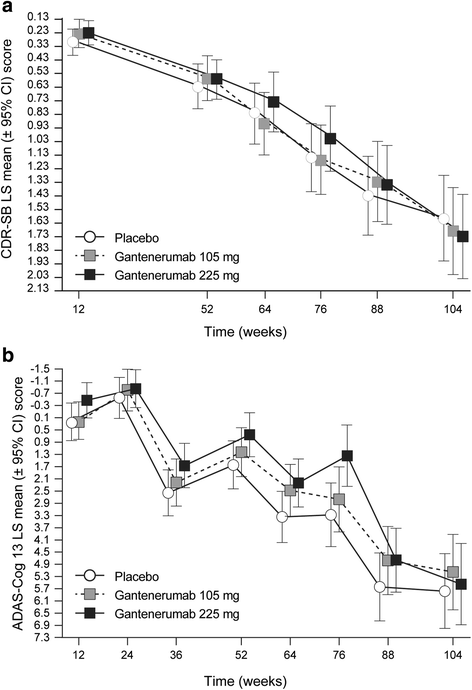
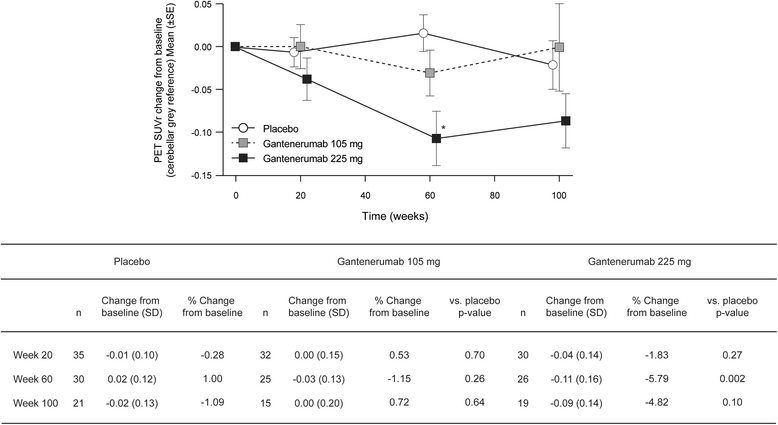
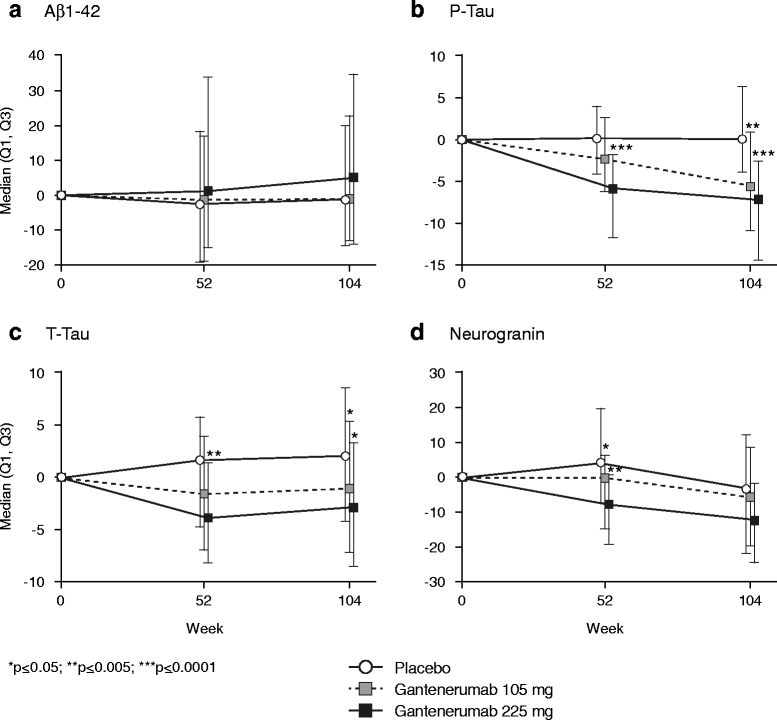
Results: Of the 3089 patients screened, 797 were randomized. The study was halted early for futility; dosing was discontinued; and the study was unblinded. No differences between groups in the primary (least squares mean [95% CI] CDR-SB change from baseline 1.60 [1.28, 1.91], 1.69 [1.37, 2.01], and 1.73 [1.42, 2.04] for placebo, gantenerumab 105 mg, and gantenerumab 225 mg, respectively) or secondary clinical endpoints were observed. The incidence of generally asymptomatic amyloid-related imaging abnormalities increased in a dose- and APOE ε4 genotype-dependent manner. Exploratory analyses suggested a dose-dependent drug effect on clinical and biomarker endpoints.
Conclusions: The study was stopped early for futility, but dose-dependent effects observed in exploratory analyses on select clinical and biomarker endpoints suggest that higher dosing with gantenerumab may be necessary to achieve clinical efficacy.
Trial registration: ClinicalTrials.gov, NCT01224106 . Registered on October 14, 2010.
Keywords: Alzheimer’s disease; Gantenerumab; SCarlet RoAD.
Conflict of interest statement
Ethics approval and consent to participate
SCarlet RoAD was approved by individual institution ethics committees or institutional review boards and was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice (GCP). Please refer to Additional file 1 for details. Written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
SO, RAL, ED, TN, EA, SR, CH, PD, GK, MA, LS, and PF are current or former employees of F. Hoffmann La-Roche and own stock or stock options in Roche. FB has received consultancy fees/honoraria from Bayer-Schering Pharma, Biogen Idec, Teva, Merck-Serono, Novartis, F. Hoffmann-La Roche, Synthon BV, Janssen Research, and Genzyme. FB has received grants from the Dutch Multiple Sclerosis Society and the European Union Seventh Framework Programme/Horizon 2020 (H2020). FB’s institution has received fees from IXICO for educational presentation development. FB is supported by the National Institute for Health Research-UCL Hospitals Biomedical Research Centre. KB reports receipt of a grant to his institution for performing CSF analyses during the conduct of the study. KB reports receiving personal fees from Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Pfizer, and Roche Diagnostics. KB is the co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. MB reports receiving grants from Elizabeth Forward School District/Lilly Mental Health and Diabetes Program (2014–2015) and from the H2020-JTI-IMI2-2015-05 (EC) (Eli Lilly and AstraZeneca) Models of Patient Engagement for Alzheimer’s Disease (MOPEAD) Project (2016–2018). KB reports receiving personal fees from Grifols, Janssen, Eli Lilly, MSD, Nutricia, Roche, and Servier. PS reports receiving speaker fees from Roche AG during the conduct of the study and consultancy fees from Axovant Sciences, Probiodrug, and EIP Pharma. BD reports receiving personal fees from Eli Lilly and grants from Pfizer.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Gantenerumab reduces amyloid-β plaques in patients with prodromal to moderate Alzheimer's disease: a PET substudy interim analysis.Alzheimers Res Ther. 2019 Dec 12;11(1):101. doi: 10.1186/s13195-019-0559-z. Alzheimers Res Ther. 2019. PMID: 31831056 Free PMC article.
-
Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer's disease (BLAZE).Alzheimers Res Ther. 2018 Sep 19;10(1):96. doi: 10.1186/s13195-018-0424-5. Alzheimers Res Ther. 2018. PMID: 30231896 Free PMC article. Clinical Trial.
-
Long-term safety of gantenerumab in participants with Alzheimer's disease: A phase III, open-label extension study (SCarlet RoAD).J Alzheimers Dis. 2025 Jan;103(2):528-541. doi: 10.1177/13872877241303644. Epub 2024 Dec 16. J Alzheimers Dis. 2025. PMID: 39686620 Clinical Trial.
-
Efficacy and safety studies of gantenerumab in patients with Alzheimer's disease.Expert Rev Neurother. 2014 Sep;14(9):973-86. doi: 10.1586/14737175.2014.945522. Epub 2014 Aug 1. Expert Rev Neurother. 2014. PMID: 25081412 Review.
-
Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer's disease with potential for near term approval.Alzheimers Res Ther. 2020 Aug 12;12(1):95. doi: 10.1186/s13195-020-00663-w. Alzheimers Res Ther. 2020. PMID: 32787971 Free PMC article. Review.
Cited by
-
Role of Retinal Amyloid-β in Neurodegenerative Diseases: Overlapping Mechanisms and Emerging Clinical Applications.Int J Mol Sci. 2021 Feb 26;22(5):2360. doi: 10.3390/ijms22052360. Int J Mol Sci. 2021. PMID: 33653000 Free PMC article. Review.
-
Quantitative systems pharmacology model of the amyloid pathway in Alzheimer's disease: Insights into the therapeutic mechanisms of clinical candidates.CPT Pharmacometrics Syst Pharmacol. 2023 Jan;12(1):62-73. doi: 10.1002/psp4.12876. Epub 2022 Nov 6. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 36281062 Free PMC article.
-
Effect of hypoglycemic events on cognitive function in individuals with type 2 diabetes mellitus: a dose-response meta-analysis.Front Neurol. 2024 Aug 13;15:1394499. doi: 10.3389/fneur.2024.1394499. eCollection 2024. Front Neurol. 2024. PMID: 39193149 Free PMC article.
-
Monoclonal antibodies and aptamers: The future therapeutics for Alzheimer's disease.Acta Pharm Sin B. 2024 Jul;14(7):2795-2814. doi: 10.1016/j.apsb.2024.03.034. Epub 2024 Apr 17. Acta Pharm Sin B. 2024. PMID: 39027235 Free PMC article. Review.
-
Shared Risk Factors between Dementia and Atherosclerotic Cardiovascular Disease.Int J Mol Sci. 2022 Aug 29;23(17):9777. doi: 10.3390/ijms23179777. Int J Mol Sci. 2022. PMID: 36077172 Free PMC article. Review.
References
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

