Does intensive management improve remission rates in patients with intermediate rheumatoid arthritis? (the TITRATE trial): study protocol for a randomised controlled trial
- PMID: 29221496
- PMCID: PMC5723045
- DOI: 10.1186/s13063-017-2330-8
Does intensive management improve remission rates in patients with intermediate rheumatoid arthritis? (the TITRATE trial): study protocol for a randomised controlled trial
Abstract
Background: Uncontrolled active rheumatoid arthritis can lead to increasing disability and reduced quality of life over time. 'Treating to target' has been shown to be effective in active established disease and also in early disease. However, there is a lack of nationally agreed treatment protocols for patients with established rheumatoid arthritis who have intermediate disease activity. This trial is designed to investigate whether intensive management of disease leads to a greater number of remissions at 12 months. Levels of disability and quality of life, and acceptability and cost-effectiveness of the intervention will also be examined.
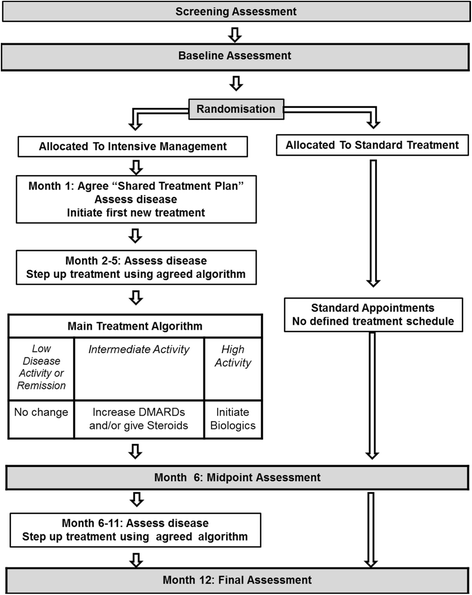
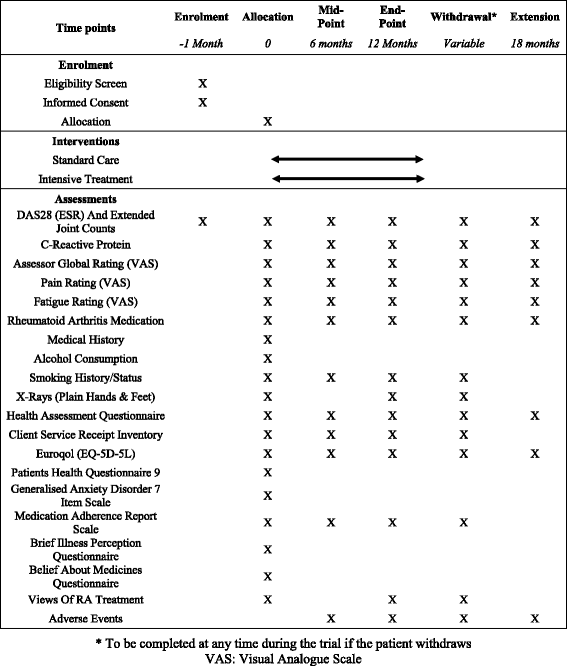
Methods: The trial is a 12-month, pragmatic, randomised, open-label, two-arm, parallel-group, multicentre trial undertaken at specialist rheumatology centres across England. Three hundred and ninety-eight patients with established rheumatoid arthritis will be recruited. They will currently have intermediate disease activity (disease activity score for 28 joints assessed using an erythrocyte sedimentation rate of 3.2 to 5.1 with at least three active joints) and will be taking at least one disease-modifying anti-rheumatic drug. Participants will be randomly selected to receive intensive management or standard care. Intensive management will involve monthly clinical reviews with a specialist health practitioner, where drug treatment will be optimised and an individualised treatment support programme delivered based on several principles of motivational interviewing to address identified problem areas, such as pain, fatigue and adherence. Standard care will follow standard local pathways and will be in line with current English guidelines from the National Institute for Health and Clinical Excellence. Patients will be assessed initially and at 6 and 12 months through self-completed questionnaires and clinical evaluation.
Discussion: The trial will establish whether the known benefits of intensive treatment strategies in active rheumatoid arthritis are also seen in patients with established rheumatoid arthritis who have moderately active disease. It will evaluate both the clinical and cost-effectiveness of intensive treatment.
Trial registration: Current Controlled Trials, ID: ISRCTN70160382 . Registered on 16 January 2014.
Keywords: Intensive treatment; Intermediate disease activity; Randomised controlled trial; Rheumatoid arthritis; Treating to target; Tumour necrosis factor inhibitors.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval for this trial was obtained from the London – West London and GTAC National Research Ethics Service (NRES) Committee (13/LO/1308). This Ethics Committee approved the study for all participating centres. All participants provide written informed consent before participating in the trial or extension study.
Consent for publication
Not applicable.
Competing interests
A list of investigators with competing interests and the details they have declared is as follows: Dr. James Galloway received consultancy fees for educational talks from UCB, Pfizer, Napp and MSD. Dr. Heidi Lempp received funding over the last 5 years from the National Institute of Health Research; European Union; Guy’s and St Thomas’ Charity, London; The Health Foundation, London; Arthritis Research UK; and the South London Membership Council. Professor Jackie Sturt received funding from Eli Lilly for consultancy activities in 2016 and will continue to do so in 2017.
Pfizer made Enbrel freely available for use outside NICE guidance in this study.
The following authors declare that they have no competing interests: Dr. Naomi Martin, Ms. Fowzia Ibrahim, Dr. Brian Tom, Professor Allan Wailoo, Mr. Jon Tosh, Ms. Louise Prothero, Dr. Sofia Georgopoulou, Professor David Scott.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Intensive therapy for moderate established rheumatoid arthritis: the TITRATE research programme.Southampton (UK): NIHR Journals Library; 2021 Aug. Southampton (UK): NIHR Journals Library; 2021 Aug. PMID: 34406722 Free Books & Documents. Review.
-
Randomised controlled trial of tumour necrosis factor inhibitors against combination intensive therapy with conventional disease-modifying antirheumatic drugs in established rheumatoid arthritis: the TACIT trial and associated systematic reviews.Health Technol Assess. 2014 Oct;18(66):i-xxiv, 1-164. doi: 10.3310/hta18660. Health Technol Assess. 2014. PMID: 25351370 Free PMC article. Clinical Trial.
-
Alternative tumour necrosis factor inhibitors (TNFi) or abatacept or rituximab following failure of initial TNFi in rheumatoid arthritis: the SWITCH RCT.Health Technol Assess. 2018 Jun;22(34):1-280. doi: 10.3310/hta22340. Health Technol Assess. 2018. PMID: 29900829 Free PMC article. Clinical Trial.
-
Tumour necrosis factor inhibitors versus combination intensive therapy with conventional disease modifying anti-rheumatic drugs in established rheumatoid arthritis: TACIT non-inferiority randomised controlled trial.BMJ. 2015 Mar 13;350:h1046. doi: 10.1136/bmj.h1046. BMJ. 2015. PMID: 25769495 Free PMC article. Clinical Trial.
-
Enzyme-linked immunosorbent assays for monitoring TNF-alpha inhibitors and antibody levels in people with rheumatoid arthritis: a systematic review and economic evaluation.Health Technol Assess. 2021 Feb;25(8):1-248. doi: 10.3310/hta25080. Health Technol Assess. 2021. PMID: 33555998 Free PMC article.
Cited by
-
The clinical effectiveness of intensive management in moderate established rheumatoid arthritis: The titrate trial.Semin Arthritis Rheum. 2020 Oct;50(5):1182-1190. doi: 10.1016/j.semarthrit.2020.07.014. Epub 2020 Jul 30. Semin Arthritis Rheum. 2020. PMID: 32931984 Free PMC article. Clinical Trial.
-
Baseline predictors of remission, pain and fatigue in rheumatoid arthritis: the TITRATE trial.Arthritis Res Ther. 2021 Nov 4;23(1):278. doi: 10.1186/s13075-021-02653-1. Arthritis Res Ther. 2021. PMID: 34736525 Free PMC article.
-
Genistein inhibits angiogenesis developed during rheumatoid arthritis through the IL-6/JAK2/STAT3/VEGF signalling pathway.J Orthop Translat. 2019 Aug 23;22:92-100. doi: 10.1016/j.jot.2019.07.007. eCollection 2020 May. J Orthop Translat. 2019. PMID: 32440504 Free PMC article.
-
A systematic review of guidelines for managing rheumatoid arthritis.BMC Rheumatol. 2019 Oct 22;3:42. doi: 10.1186/s41927-019-0090-7. eCollection 2019. BMC Rheumatol. 2019. PMID: 31660534 Free PMC article.
-
The Effectiveness of Psychological Interventions for Rheumatoid Arthritis (RA): A Systematic Review and Meta-Analysis.Life (Basel). 2023 Mar 21;13(3):849. doi: 10.3390/life13030849. Life (Basel). 2023. PMID: 36984004 Free PMC article. Review.
References
-
- Pincus T, Castrejón I, Bergman MJ, Yazici Y. Treat-to-target: not as simple as it appears. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S10–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical