GLP-1 receptor agonists: differentiation within the class
- PMID: 29221658
- PMCID: PMC5988583
- DOI: 10.1016/S2213-8587(17)30413-8
GLP-1 receptor agonists: differentiation within the class
Figures
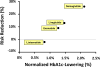
Normalized HbA1c-lowering for short-acting exenatide/BYETTA was defined as 1.00%.
Normalized HbA1c-lowering for lixisenatide was calculated based on head-to-head comparative efficacy data obtained in the GetGoal-X study. Observed HbA1c-lowering was 0.96% for short-acting exenatide/BYETTA and 0.79% for lixisenatide, corresponding to an Efficacy Ratio of 0.82 (=0.79% / 0.96%). A normalized HbA1c-lowering for lixisenatide of 0.82% was calculated by multiplying the Efficacy Ratio (0.82) times the normalized HbA1c-lowering for short-acting exenatide (1.00%).
Normalized HbA1c-lowering for long-acting exenatide/BYDUREON was calculated in a similar fashion based on comparative efficacy data in the DURATION-1 study. This yielded a value of 1.27% as the normalized HbA1c-lowering for long-acting exenatide [BYETTA].
The DURATION-6 and SUSTAIN-3 studies reported Efficacy Ratios of 1.16 and 1.67 for liraglutide and semaglutide, respectively, relative to extended-release exenatide/BYDUREON. These calculations yield values for normalized HbA1c-lowering of 1.47% and 2.12% for liraglutide and semaglutide, respectively.
The AWARD-6 and HARMONY-7 studies reported Efficacy Ratios of 1.04 and 0.79 for dulaglutide and albiglutide, respectively, relative to liraglutide. These calculations yield values for normalized HbA1c-lowering of 1.53% and 1.16% for dulaglutide and albiglutide, respectively.
Comment in
-
GLP-1 receptor agonists and cardiovascular disease: drug-specific or class effects?Lancet Diabetes Endocrinol. 2019 Feb;7(2):89-90. doi: 10.1016/S2213-8587(18)30351-6. Lancet Diabetes Endocrinol. 2019. PMID: 30683221 No abstract available.
Comment on
-
Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis.Lancet Diabetes Endocrinol. 2018 Feb;6(2):105-113. doi: 10.1016/S2213-8587(17)30412-6. Epub 2017 Dec 6. Lancet Diabetes Endocrinol. 2018. PMID: 29221659
References
-
- Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with GLP-1 receptor agonists: A meta-analysis. Lancet Diabetes Endocrinol. 2017 in press. - PubMed
-
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834–44. - PubMed
-
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

