Targeted disruption of the Kcnj5 gene in the female mouse lowers aldosterone levels
- PMID: 29222092
- PMCID: PMC6365593
- DOI: 10.1042/CS20171285
Targeted disruption of the Kcnj5 gene in the female mouse lowers aldosterone levels
Abstract
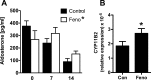
Aldosterone is released from adrenal zona glomerulosa (ZG) cells and plays an important role in Na and K homoeostasis. Mutations in the human inwardly rectifying K channel CNJ type (KCNJ) 5 (KCNJ5) gene encoding the G-coupled inwardly rectifying K channel 4 (GIRK4) cause abnormal aldosterone secretion and hypertension. To better understand the role of wild-type (WT) GIRK4 in regulating aldosterone release, we have looked at aldosterone secretion in a Kcnj5 knockout (KO) mouse. We found that female but not male KO mice have reduced aldosterone levels compared with WT female controls, but higher levels of aldosterone after angiotensin II (Ang-II) stimulation. These differences could not be explained by sex differences in aldosterone synthase (Cyp11B2) gene expression in the mouse adrenal. Using RNAseq analysis to compare WT and KO adrenals, we showed that females also have a much larger set of differentially expressed adrenal genes than males (395 compared with 7). Ingenuity Pathway Analysis (IPA) of this gene set suggested that peroxisome proliferator activated receptor (PPAR) nuclear receptors regulated aldosterone production and altered signalling in the female KO mouse, which could explain the reduced aldosterone secretion. We tested this hypothesis in H295R adrenal cells and showed that the selective PPARα agonist fenofibrate can stimulate aldosterone production and induce Cyp11b2. Dosing mice in vivo produced similar results. Together our data show that Kcnj5 is important for baseline aldosterone secretion, but its importance is sex-limited at least in the mouse. It also highlights a novel regulatory pathway for aldosterone secretion through PPARα that may have translational potential in human hyperaldosteronism.
Keywords: GIRK4 K channel; KCNJ5 gene; RNAseq; aldosterone; knockout mice.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures





Similar articles
-
Regulation of aldosterone biosynthesis by the Kir3.4 (KCNJ5) potassium channel.Clin Exp Pharmacol Physiol. 2013 Dec;40(12):895-901. doi: 10.1111/1440-1681.12151. Clin Exp Pharmacol Physiol. 2013. PMID: 23829355 Free PMC article. Review.
-
KCNJ5 mutations in aldosterone producing adenoma and relationship with adrenal cortex remodeling.Mol Cell Endocrinol. 2013 May 22;371(1-2):221-7. doi: 10.1016/j.mce.2013.01.018. Epub 2013 Jan 30. Mol Cell Endocrinol. 2013. PMID: 23376008
-
Human adrenal glomerulosa cells express K2P and GIRK potassium channels that are inhibited by ANG II and ACTH.Am J Physiol Cell Physiol. 2021 Jul 1;321(1):C158-C175. doi: 10.1152/ajpcell.00118.2021. Epub 2021 May 26. Am J Physiol Cell Physiol. 2021. PMID: 34038243 Free PMC article.
-
Visinin-like 1 is upregulated in aldosterone-producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis.Hypertension. 2012 Apr;59(4):833-9. doi: 10.1161/HYPERTENSIONAHA.111.188532. Epub 2012 Feb 13. Hypertension. 2012. PMID: 22331379
-
Minireview: potassium channels and aldosterone dysregulation: is primary aldosteronism a potassium channelopathy?Endocrinology. 2014 Jan;155(1):47-55. doi: 10.1210/en.2013-1733. Epub 2013 Dec 20. Endocrinology. 2014. PMID: 24248457 Free PMC article. Review.
Cited by
-
Cushing Syndrome in a Pediatric Patient With a KCNJ5 Variant and Successful Treatment With Low-dose Ketoconazole.J Clin Endocrinol Metab. 2021 May 13;106(6):1606-1616. doi: 10.1210/clinem/dgab118. J Clin Endocrinol Metab. 2021. PMID: 33630995 Free PMC article.
-
Primary Aldosteronism: Metabolic Reprogramming and the Pathogenesis of Aldosterone-Producing Adenomas.Cancers (Basel). 2021 Jul 23;13(15):3716. doi: 10.3390/cancers13153716. Cancers (Basel). 2021. PMID: 34359615 Free PMC article.
-
The ex vivo perfused mouse adrenal gland-a new model to study aldosterone secretion.Pflugers Arch. 2024 Jun;476(6):911-922. doi: 10.1007/s00424-024-02950-z. Epub 2024 Mar 28. Pflugers Arch. 2024. PMID: 38538989 Free PMC article.
-
DNA Methylation of the Angiotensinogen Gene, AGT, and the Aldosterone Synthase Gene, CYP11B2 in Cardiovascular Diseases.Int J Mol Sci. 2021 Apr 27;22(9):4587. doi: 10.3390/ijms22094587. Int J Mol Sci. 2021. PMID: 33925539 Free PMC article. Review.
-
Methionine supplementation regulates eggshell quality and uterine transcriptome in late-stage broiler breeders.Anim Nutr. 2024 Jul 17;19:56-69. doi: 10.1016/j.aninu.2024.04.026. eCollection 2024 Dec. Anim Nutr. 2024. PMID: 39628644 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

