Effect of Start-Up Strategies and Electrode Materials on Carbon Dioxide Reduction on Biocathodes
- PMID: 29222104
- PMCID: PMC5795077
- DOI: 10.1128/AEM.02242-17
Effect of Start-Up Strategies and Electrode Materials on Carbon Dioxide Reduction on Biocathodes
Abstract
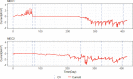
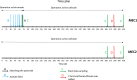
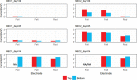
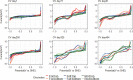
The enrichment of CO2-reducing microbial biocathodes is challenging. Previous research has shown that a promising approach could be to first enrich bioanodes and then lower the potential so the electrodes are converted into biocathodes. However, the effect of such a transition on the microbial community on the electrode has not been studied. The goal of this study was thus to compare the start-up of biocathodes from preenriched anodes with direct start-up from bare electrodes and to investigate changes in microbial community composition. The effect of three electrode materials on the long-term performance of the biocathodes was also investigated. In this study, preenrichment of acetate-oxidizing bioanodes did not facilitate the start-up of biocathodes. It took about 170 days for the preenriched electrodes to generate substantial cathodic current, compared to 83 days for the bare electrodes. Graphite foil and carbon felt cathodes produced higher current at the beginning of the experiment than did graphite rods. However, all electrodes produced similar current densities at the end of the over 1-year-long study (2.5 A/m2). Methane was the only product detected during operation of the biocathodes. Acetate was the only product detected after inhibition of the methanogens. Microbial community analysis showed that Geobacter sp. dominated the bioanodes. On the biocathodes, the Geobacter sp. was succeeded by Methanobacterium spp., which made up more than 80% of the population. After inhibition of the methanogens, Acetobacterium sp. became dominant on the electrodes (40% relative abundance). The results suggested that bioelectrochemically generated H2 acted as an electron donor for CO2 reduction.IMPORTANCE In microbial electrochemical systems, living microorganisms function as catalysts for reactions on the anode and/or the cathode. There is a variety of potential applications, ranging from wastewater treatment and biogas generation to production of chemicals. Systems with biocathodes could be used to reduce CO2 to methane, acetate, or other high-value chemicals. The technique can be used to convert solar energy to chemicals. However, enriching biocathodes that are capable of CO2 reduction is more difficult and less studied than enriching bioanodes. The effect of different start-up strategies and electrode materials on the microbial communities that are enriched on biocathodes has not been studied. The purpose of this study was to investigate two different start-up strategies and three different electrode materials for start-up and long-term operation of biocathodes capable of reducing CO2 to valuable biochemicals.
Keywords: acetogens; biocathode; cyclic voltammetry; methanogens; microbial community structure; microbial electrolysis cells; start-up strategies.
Copyright © 2018 Saheb-Alam et al.
Figures


References
-
- Modin O, Aulenta F. 2017. Three promising applications of microbial electrochemistry for the water sector. Environ Sci 3:391–402. doi: 10.1039/C6EW00325G. - DOI
-
- Rozendal RA, Hamelers HVM, Euverink GJW, Metz SJ, Buisman CJN. 2006. Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int J Hydrogen Energy 31:1632–1640. doi: 10.1016/j.ijhydene.2005.12.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

