Nanoscale Tuning of VCAM-1 Determines VLA-4-Dependent Melanoma Cell Plasticity on RGD Motifs
- PMID: 29222169
- PMCID: PMC5837006
- DOI: 10.1158/1541-7786.MCR-17-0272
Nanoscale Tuning of VCAM-1 Determines VLA-4-Dependent Melanoma Cell Plasticity on RGD Motifs
Abstract
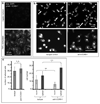
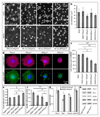
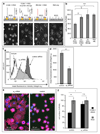
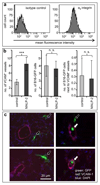
The biophysical fine-tuning of cancer cell plasticity is crucial for tumor progression but remains largely enigmatic. Although vascular cell adhesion molecule-1 (VCAM-1/CD106) has been implicated in melanoma progression, here its presentation on endothelial cells was associated with diminished melanoma cell spreading. Using a specific nanoscale modulation of VCAM-1 (tunable from 70 to 670 ligands/μm²) next to integrin ligands (RGD motifs) in a bifunctional system, reciprocal regulation of integrin α4 (ITGA4/VLA-4/CD49d)-dependent adhesion and spreading of melanoma cells was found. As the VCAM-1/VLA-4 receptor pair facilitated adhesion, while at the same time antagonizing RGD-mediated spreading, melanoma cell morphogenesis on these bifunctional matrices was directly regulated by VCAM-1 in a dichotomic and density-dependent fashion. This was accompanied by concordant regulation of F-actin cytoskeleton remodeling, Rac1-expression, and paxillin-related adhesion formation. The novel function of VCAM-1 was corroborated in vivo using two murine models of pulmonary metastasis. The regulation of melanoma cell plasticity by VCAM-1 highlights the complex regulation of tumor-matrix interactions.Implications: Nanotechnology has revealed a novel dichotomic function of the VCAM-1/VLA-4 interaction on melanoma cell plasticity, as nanoscale tuning of this interaction reciprocally determines adhesion and spreading in a ligand density-dependent manner. Mol Cancer Res; 16(3); 528-42. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures


References
-
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. - PubMed
-
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. - PubMed
-
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

