Non-chemotherapy drug-induced neutropenia: key points to manage the challenges
- PMID: 29222255
- PMCID: PMC6142577
- DOI: 10.1182/asheducation-2017.1.187
Non-chemotherapy drug-induced neutropenia: key points to manage the challenges
Abstract
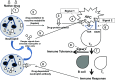
Non-chemotherapy idiosyncratic drug-induced neutropenia (IDIN) is a relatively rare but potentially fatal disorder that occurs in susceptible individuals, with an incidence of 2.4 to 15.4 cases per million population. Affected patients typically experience severe neutropenia within several weeks to several months after first exposure to a drug, and mortality is ∼5%. The drugs most frequently associated with IDIN include metamizole, clozapine, sulfasalazine, thiamazole, carbimazole, amoxicillin, cotrimoxazole, ticlopidine, and valganciclovir. The idiosyncratic nature of IDIN, the lack of mouse models and diagnostic testing, and its low overall incidence make rigorous studies to elucidate possible mechanisms exceptionally difficult. An immune mechanism for IDIN involving neutrophil destruction by hapten (drug)-specific antibodies and drug-induced autoantibodies is frequently suggested, but strong supporting evidence is lacking. Although laboratory testing for neutrophil drug-dependent antibodies is rarely performed because of the complexity and low sensitivity of tests currently in use, these assays could possibly be enhanced by using reactive drug metabolites in place of the parent drug. Patients typically experience acute, severe neutropenia, or agranulocytosis (<0.5 × 109 neutrophils/L) and symptoms of fever, chills, sore throat, and muscle and joint pain. Diagnosis can be difficult, but timely recognition is critical because if left untreated, there is an increase in mortality. Expanded studies of the production and mechanistic role of reactive drug metabolites, genetic associations, and improved animal models of IDIN are essential to further our understanding of this important disorder.
© 2016 by The American Society of Hematology. All rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The author is on the Board of Directors or an advisory committee and has consulted for Ionis Pharmaceuticals.
Figures

References
-
- Uetrecht J. Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol. 2007;47:513-539. - PubMed
-
- Thompson RA, Isin EM, Ogese MO, Mettetal JT, Williams DP. Reactive metabolites: current and emerging risk and hazard assessments. Chem Res Toxicol. 2016;29(4):505-533. - PubMed
-
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228-237. - PubMed
-
- Johnston A, Uetrecht J. Current understanding of the mechanisms of idiosyncratic drug-induced agranulocytosis. Expert Opin Drug Metab Toxicol. 2015;11(2):243-257. - PubMed
-
- Medrano-Casique N, Tong HY, Borobia AM, Carcas AJ, Frías J, Ramírez E. Non-chemotherapy-induced agranulocytosis detected by a prospective pharmacovigilance program in a tertiary hospital. Basic Clin Pharmacol Toxicol. 2015;117(6):399-408. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

