How should we sequence and combine novel therapies in CLL?
- PMID: 29222277
- PMCID: PMC6142575
- DOI: 10.1182/asheducation-2017.1.346
How should we sequence and combine novel therapies in CLL?
Abstract
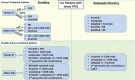
With the recent approval of several effective and well-tolerated novel agents (NAs), including ibrutinib, idelalisib, venetoclax, and obinutuzumab, patients with chronic lymphocytic leukemia (CLL) have more therapeutic options than ever before. The availability of these agents is both an important advance for patients but also a challenge for practicing hematologist/oncologists to learn how best to sequence NAs, both with respect to chemoimmunotherapy (CIT) and to other NAs. The sequencing of NAs in clinical practice should be guided both by an individual patient's prognostic markers, such as FISH and immunoglobulin heavy chain variable region (IGHV)-mutation status, as well as the patient's medical comorbidities and goals of care. For older, frailer patients with lower-risk CLL prognostic markers, NA monotherapy may remain a mainstay of CLL treatment for years to come. For younger, fitter patients and those with higher-risk CLL, such as del(17p) or unmutated IGHV, combination approaches may prove to be more valuable than NA monotherapy. Trials are currently evaluating the efficacy of several such combination approaches, including NA plus anti-CD20 monoclonal antibody, NA plus NA (with or without anti-CD20 monoclonal antibody), and NA plus CIT. Given the tremendous efficacy of the already approved NAs, as well as the promising data for next generation NAs, the development of well-tolerated, highly effective combination strategies with curative potential for patients with CLL has become a realistic goal.
© 2016 by The American Society of Hematology. All rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.S.D. is on the Board of Directors or an advisory committee for TG Therapeutics, Genentech, Pharmacyclics, Janssen, Abbvie, Gilead, and InCyte; has received research funding from TG Therapeutics, Genentech, Pharmacyclics, and Infinity; and has consulted for Janssen, TG Therapeutics, Genentech, Astra–Zeneca, Merck, Celgene, Abbvie, and Pharmacyclics.
Figures

References
-
- Fischer K, Bahlo J, Fink AM, et al. . Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208-215. - PubMed
-
- Eichhorst B, Fink AM, Bahlo J, et al. ; international group of investigators; German CLL Study Group (GCLLSG). First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928-942. - PubMed
-
- Hutchinson JH. Congenital agammaglobulinaemia. Lancet. 1955;269(6895):844-847. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

