Utilization of blood by-products: An in silico and experimental combined study for BSA usage
- PMID: 29222431
- PMCID: PMC5722935
- DOI: 10.1038/s41598-017-17029-2
Utilization of blood by-products: An in silico and experimental combined study for BSA usage
Abstract
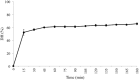
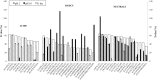
In order to exploit industrial discards, protein enzymatic hydrolysis is a currently popular methodology for obtaining bioactive peptides. However, once released, most promising peptides have to be selected from the mixture. In this work, the suitability of pepsin (EC 3.4.23.1) to hydrolyse serum albumin in order to obtain bioactive peptides was assessed. Then, a suitable process to obtain best separation of bioactive peptides was evaluated, using polyethersulfone membranes at different pH values. Serum albumin was easily hydrolysed by pepsin, reaching a DH value of the 65.64 ± 1.57% of the maximum possible. A 23.25% of the identified peptides possessed high bioactivity scores (greater than 0.5), and one of them had reported bioactivity (LLL). Charge mechanisms always predominated over the sieve effect, and best transmission was accomplished at pH values close to the peptides isoelectric points. Basic and neutral peptides with the highest scores were always the most transmitted. Membrane material had greater influence than NMWCO in determining peptide transmission. In order to obtain purified fractions rich in peptides with high bioactivity scores from serum albumin, polyethersulfone membranes (applicable to industrial scale) of 5 kDa MWCO should be used at basic pH values after pepsin digestion.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Influence of pH on the appearance of active peptides in the course of peptic hydrolysis of bovine haemoglobin.Prep Biochem Biotechnol. 2005;35(2):85-102. doi: 10.1081/PB-200054693. Prep Biochem Biotechnol. 2005. PMID: 15881591
-
Protein acidification and hydrolysis by pepsin ensure efficient trypsin-catalyzed hydrolysis.Food Funct. 2021 May 21;12(10):4570-4581. doi: 10.1039/d1fo00413a. Epub 2021 Apr 28. Food Funct. 2021. PMID: 33908536
-
Hydrolysis of beta-casein by gastric proteases. I. Comparison of proteolytic action of bovine chymosin and pepsin A.Int J Pept Protein Res. 1991 Jun;37(6):494-501. Int J Pept Protein Res. 1991. PMID: 1917306
-
Revisiting the enzymatic kinetics of pepsin using isothermal titration calorimetry.Food Chem. 2018 Dec 1;268:94-100. doi: 10.1016/j.foodchem.2018.06.042. Epub 2018 Jun 15. Food Chem. 2018. PMID: 30064809
-
Enhancing bioactive peptide release and identification using targeted enzymatic hydrolysis of milk proteins.Anal Bioanal Chem. 2018 Jun;410(15):3407-3423. doi: 10.1007/s00216-017-0793-9. Epub 2017 Dec 19. Anal Bioanal Chem. 2018. PMID: 29260283 Review.
References
-
- Li-Chan EC. Bioactive peptides and protein hydrolysates: research trends and challenges for application as nutraceuticals and functional food ingredients. Current Opinion in Food Science. 2015;1:28–37. doi: 10.1016/j.cofs.2014.09.005. - DOI
-
- Korhonen H, Pihlanto A. Bioactive peptides: production and functionality. International dairy journal. 2006;16:945–960. doi: 10.1016/j.idairyj.2005.10.012. - DOI
-
- Fernández A, Riera FA. Membrane Fractionation of a β-Lactoglobulin Tryptic Digest: Effect of the Hydrolysate Concentration. Industrial & Engineering Chemistry Research. 2012;51:15738–15744. doi: 10.1021/ie302376g. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

