Metabolic versatility of small archaea Micrarchaeota and Parvarchaeota
- PMID: 29222443
- PMCID: PMC5864196
- DOI: 10.1038/s41396-017-0002-z
Metabolic versatility of small archaea Micrarchaeota and Parvarchaeota
Abstract
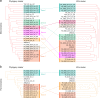
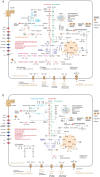
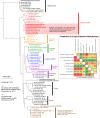
Small acidophilic archaea belonging to Micrarchaeota and Parvarchaeota phyla are known to physically interact with some Thermoplasmatales members in nature. However, due to a lack of cultivation and limited genomes on hand, their biodiversity, metabolisms, and physiologies remain largely unresolved. Here, we obtained 39 genomes from acid mine drainage (AMD) and hot spring environments around the world. 16S rRNA gene based analyses revealed that Parvarchaeota were only detected in AMD and hot spring habitats, while Micrarchaeota were also detected in others including soil, peat, hypersaline mat, and freshwater, suggesting a considerable higher diversity and broader than expected habitat distribution for this phylum. Despite their small genomes (0.64-1.08 Mb), these archaea may contribute to carbon and nitrogen cycling by degrading multiple saccharides and proteins, and produce ATP via aerobic respiration and fermentation. Additionally, we identified several syntenic genes with homology to those involved in iron oxidation in six Parvarchaeota genomes, suggesting their potential role in iron cycling. However, both phyla lack biosynthetic pathways for amino acids and nucleotides, suggesting that they likely scavenge these biomolecules from the environment and/or other community members. Moreover, low-oxygen enrichments in laboratory confirmed our speculation that both phyla are microaerobic/anaerobic, based on several specific genes identified in them. Furthermore, phylogenetic analyses provide insights into the close evolutionary history of energy related functionalities between both phyla with Thermoplasmatales. These results expand our understanding of these elusive archaea by revealing their involvement in carbon, nitrogen, and iron cycling, and suggest their potential interactions with Thermoplasmatales on genomic scale.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

