Elastic Fibers and Large Artery Mechanics in Animal Models of Development and Disease
- PMID: 29222533
- PMCID: PMC5816253
- DOI: 10.1115/1.4038704
Elastic Fibers and Large Artery Mechanics in Animal Models of Development and Disease
Abstract
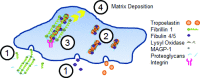
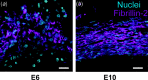
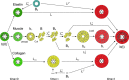
Development of a closed circulatory system requires that large arteries adapt to the mechanical demands of high, pulsatile pressure. Elastin and collagen uniquely address these design criteria in the low and high stress regimes, resulting in a nonlinear mechanical response. Elastin is the core component of elastic fibers, which provide the artery wall with energy storage and recoil. The integrity of the elastic fiber network is affected by component insufficiency or disorganization, leading to an array of vascular pathologies and compromised mechanical behavior. In this review, we discuss how elastic fibers are formed and how they adapt in development and disease. We discuss elastic fiber contributions to arterial mechanical behavior and remodeling. We primarily present data from mouse models with elastic fiber deficiencies, but suggest that alternate small animal models may have unique experimental advantages and the potential to provide new insights. Advanced ultrastructural and biomechanical data are constantly being used to update computational models of arterial mechanics. We discuss the progression from early phenomenological models to microstructurally motivated strain energy functions for both collagen and elastic fiber networks. Although many current models individually account for arterial adaptation, complex geometries, and fluid-solid interactions (FSIs), future models will need to include an even greater number of factors and interactions in the complex system. Among these factors, we identify the need to revisit the role of time dependence and axial growth and remodeling in large artery mechanics, especially in cardiovascular diseases that affect the mechanical integrity of the elastic fibers.
Figures



References
-
- Schwartz, S. M. , and Benditt, E. P. , 1972, “ Studies on Aortic Intima—I: Structure and Permeability of Rat Thoracic Aortic Intima,” Am. J. Pathol., 66(2), pp. 241–264.https://www.ncbi.nlm.nih.gov/pubmed/5009972 - PMC - PubMed
-
- Gerrity, R. G. , Richardson, M. , Somer, J. B. , Bell, F. P. , and Schwartz, C. J. , 1977, “ Endothelial Cell Morphology in Areas of In Vivo Evans Blue Uptake in the Aorta of Young Pigs—II: Ultrastructure of the Intima in Areas of Differing Permeability to Proteins,” Am. J. Pathol., 89(2), pp. 313–334.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2032231/ - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

