Diolistic Labeling and Analysis of Dendritic Spines
- PMID: 29222782
- PMCID: PMC6112159
- DOI: 10.1007/978-1-4939-7571-6_14
Diolistic Labeling and Analysis of Dendritic Spines
Abstract
Dendritic spines are diverse and plastic components of the neuronal cell apparatus and are highly responsive to trophic factors during both development and adulthood. Diolistic labeling of neurons with lipophilic fluorescent dyes, coupled with advanced high-resolution microscopy methods, provides robust labeling of dendritic spines for assessment of their density and morphology. Here, we describe a method for labeling of dendritic spines using diolistic labeling in ex vivo brain slices, visualization using confocal laser scanning microscopy, deconvolution, and analysis using the Surpass module of Bitplane Imaris software.
Keywords: 3-D reconstruction; Confocal imaging; Deconvolution; Dendritic spine; Diolistic; Lipophilic dye; Morphology.
Figures

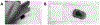





















References
-
- Cho C, MacDonald R, Shang J, Cho MJ, Chalifour LE, Paudel HK (2017) Early growth response-1-mediated down-regulation of drebrin correlates with loss of dendritic spines. J Neurochem, in press. - PubMed
-
- Andero R, Choi DC, Ressler KJ (2014) BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci 122:169–192. - PubMed
-
- Gomez-Palacio-Schjetnan A, Escobar ML (2013) Neurotrophins and synaptic plasticity. Curr Top Behav Neurosci 15:117–136. - PubMed
-
- Irala D, Bonafina A, Fontanet PA, Alsina FC, Paratcha G, Ledda F (2016) The GDNF-GFRalpha1 complex promotes the development of hippocampal dendritic arbors and spines via NCAM. Development 143 (22):4224–4235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

