Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts
- PMID: 29222823
- PMCID: PMC6334527
- DOI: 10.1111/bpa.12583
Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts
Abstract
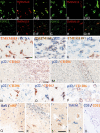
Inflammatory mechanisms, involving granulocytes, T-cells, B-cells, macrophages and activated microglia, have been suggested to play a pathogenic role in experimental models of stroke and may be targets for therapeutic intervention. However, knowledge on the inflammatory response in human stroke lesions is limited. Here, we performed a quantitative study on the inflammatory reaction in human ischemic infarct lesions. We found increased numbers of T-lymphocytes, mainly CD8+ cells, but not of B-lymphocytes. Their number was very low in comparison to that seen in inflammatory diseases of the central nervous system and they did not show signs of activation. Polymorphonuclear leukocytes were present in meninges and less prominently in the perivascular space in early lesions, but their infiltration into the lesioned tissue was sparse with the exception of a single case. Microglia were lost in the necrotic core of fresh lesions, their number was increased in the surrounding penumbra, apparently due to proliferation. Using TMEM119 as a marker for the resident microglia pool, macrophages in lesions were in part derived from the original microglia pool, depending on the lesion stage. Most microglia and macrophages revealed a pro-inflammatory activation pattern, expressing molecules involved in phagocytosis, oxidative injury, antigen presentation and iron metabolism and had partially lost the expression of P2RY12, an antigen expressed on homeostatic ("resting") microglia in rodents. At later lesion stages, the majority of macrophages showed intermediate activation patterns, expressing pro-inflammatory and anti-inflammatory markers. Microglia in the normal white matter of controls and stroke patients were already partly activated toward a pro-inflammatory phenotype. Our data suggest that the direct contribution of lymphocytes and granulocytes to active tissue injury in human ischemic infarct lesions is limited and that stroke therapy that targets pro-inflammatory microglia and macrophage activation may be effective.
Keywords: P2RY12; Stroke; TMEM119; granulocytes; lymphocytes; macrophages; microglia.
© 2017 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors have no conflict of interest.
Figures





References
-
- Aboul‐Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Bruck W et al (2003) Preferential loss of myelin‐associated glycoprotein reflects hypoxia‐like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol 62:25–33. - PubMed
-
- Bauer J, Lassmann H (2016) Neuropathological techniques to investigate central nervous system sections in multiple sclerosis. Methods Mol Biol 1304:211–229. - PubMed
-
- Bauer J, Stadelmann C, Bancher C, Jellinger K, Lassmann H (1999) Apoptosis of T lymphocytes in acute disseminated encephalomyelitis. Acta Neuropathol 97:543–546. - PubMed
-
- Booss J, Esiri MM, Tourtellotte WW, Mason DY (1983) Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci 62:219–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

