Durability of quality of life benefits of transcatheter aortic valve replacement: Long-term results from the CoreValve US extreme risk trial
- PMID: 29223434
- PMCID: PMC5821894
- DOI: 10.1016/j.ahj.2017.08.006
Durability of quality of life benefits of transcatheter aortic valve replacement: Long-term results from the CoreValve US extreme risk trial
Abstract
Background: For patients with severe aortic stenosis (AS) at extreme surgical risk, transcatheter aortic valve replacement (TAVR) leads to improved survival and health status when compared with medical therapy. Whether the early health status benefits of TAVR in these patients are sustained beyond 1 year of follow-up is unknown.
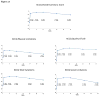
Methods and results: Six hundred thirty-nine patients with severe AS at extreme surgical risk underwent TAVR in the CoreValve US Extreme Risk Pivotal trial. Health status was evaluated at baseline and at 1, 6, 12, 24, and 36 months using the Kansas City Cardiomyopathy Questionnaire (KCCQ), the Short-Form-12, and the EuroQoL-5D. Analyses were performed using pattern mixture models to account for both death and missing data and were stratified by iliofemoral (IF) and non-iliofemoral (non-IF) access. After TAVR, there was substantial health status improvement in disease-specific and generic scales by 6 to 12 months. Although there were small declines in health status after 12 months, the initial benefits of TAVR were largely sustained through 3 years for both IF and non-IF cohorts (change from baseline in KCCQ Overall Summary score 19.0 points in IF patients and 14.9 points in non-IF patients; P<.01 for both comparisons). Among surviving patients, clinically meaningful (≥10 point) improvements in the KCCQ Overall Summary Score at 3 years were observed in 85.0% and 83.4% of IF and non-IF patients respectively.
Conclusions: Among extreme risk patients with severe AS, TAVR resulted in large initial health status benefits that were sustained through 3-year follow-up. Although late mortality was high in this population, these findings demonstrate that TAVR offers substantial and durable health status improvements for surviving patients.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





References
-
- Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, Hermiller J, Jr, Hughes GC, Harrison JK, Coselli J, Diez J, Kafi A, Schreiber T, Gleason TG, Conte J, Buchbinder M, Deeb GM, Carabello B, Serruys PW, Chenoweth S, Oh JK CoreValve United States Clinical I. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–81. - PubMed
-
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S Investigators PT. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. - PubMed
-
- Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB Investigators PT. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–704. - PubMed
-
- Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH, Babaliaros VC, Herrmann HC, Szeto WY, Pichard AD, Williams MR, Fontana GP, Miller DC, Anderson WN, Akin JJ, Davidson MJ, Smith CR investigators Pt. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–91. - PubMed
-
- Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, Vidaillet H, Phillips RS. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project. JAMA. 1998;279:371–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

