Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation
- PMID: 29223828
- PMCID: PMC7505164
- DOI: 10.1016/j.ctrv.2017.11.008
Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation
Abstract
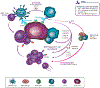
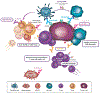
Immunoglobulin (Ig) G1 antibodies stimulate antibody-dependent cell-mediated cytotoxicity (ADCC). Cetuximab, an IgG1 isotype monoclonal antibody, is a standard-of-care treatment for locally advanced and recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) and metastatic colorectal cancer (CRC). Here we review evidence regarding the clinical relevance of cetuximab-mediated ADCC and other immune functions and provide a biological rationale concerning why this property positions cetuximab as an ideal partner for immune checkpoint inhibitors (ICIs) and other emerging immunotherapies. We performed a nonsystematic review of available preclinical and clinical data involving cetuximab-mediated immune activity and combination approaches of cetuximab with other immunotherapies, including ICIs, in SCCHN and CRC. Indeed, cetuximab mediates ADCC activity in the intratumoral space and primes adaptive and innate cellular immunity. However, counterregulatory mechanisms may lead to immunosuppressive feedback loops. Accordingly, there is a strong rationale for combining ICIs with cetuximab for the treatment of advanced tumors, as targeting CTLA-4, PD-1, and PD-L1 can ostensibly overcome these immunosuppressive counter-mechanisms in the tumor microenvironment. Moreover, combining ICIs (or other immunotherapies) with cetuximab is a promising strategy for boosting immune response and enhancing response rates and durability of response. Cetuximab immune activity-including, but not limited to, ADCC-provides a strong rationale for its combination with ICIs or other immunotherapies to synergistically and fully mobilize the adaptive and innate immunity against tumor cells. Ongoing prospective studies will evaluate the clinical effect of these combination regimens and their immune effect in CRC and SCCHN and in other indications.
Keywords: ADCC; CTLA-4; Cetuximab; Immunotherapy; PD-1; PD-L1.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Similar articles
-
Anti-EGFR Targeted Monoclonal Antibody Isotype Influences Antitumor Cellular Immunity in Head and Neck Cancer Patients.Clin Cancer Res. 2016 Nov 1;22(21):5229-5237. doi: 10.1158/1078-0432.CCR-15-2971. Epub 2016 May 23. Clin Cancer Res. 2016. PMID: 27217441 Free PMC article.
-
Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers.J Immunother Cancer. 2018 Jul 3;6(1):65. doi: 10.1186/s40425-018-0376-0. J Immunother Cancer. 2018. PMID: 29970158 Free PMC article. Review.
-
Tumor microenvironmental modification by the current target therapy for head and neck squamous cell carcinoma.J Exp Clin Cancer Res. 2023 May 5;42(1):114. doi: 10.1186/s13046-023-02691-4. J Exp Clin Cancer Res. 2023. PMID: 37143088 Free PMC article. Review.
-
Tumor-Specific Antibody, Cetuximab, Enhances the In Situ Vaccine Effect of Radiation in Immunologically Cold Head and Neck Squamous Cell Carcinoma.Front Immunol. 2020 Nov 12;11:591139. doi: 10.3389/fimmu.2020.591139. eCollection 2020. Front Immunol. 2020. PMID: 33281820 Free PMC article.
-
The Right Partner in Crime: Unlocking the Potential of the Anti-EGFR Antibody Cetuximab via Combination With Natural Killer Cell Chartering Immunotherapeutic Strategies.Front Immunol. 2021 Sep 7;12:737311. doi: 10.3389/fimmu.2021.737311. eCollection 2021. Front Immunol. 2021. PMID: 34557197 Free PMC article. Review.
Cited by
-
Pharmacogenetics Role of Genetic Variants in Immune-Related Factors: A Systematic Review Focusing on mCRC.Pharmaceutics. 2022 Nov 15;14(11):2468. doi: 10.3390/pharmaceutics14112468. Pharmaceutics. 2022. PMID: 36432658 Free PMC article. Review.
-
Beyond Microsatellite Instability: Evolving Strategies Integrating Immunotherapy for Microsatellite Stable Colorectal Cancer.Curr Treat Options Oncol. 2021 Jun 10;22(8):69. doi: 10.1007/s11864-021-00870-z. Curr Treat Options Oncol. 2021. PMID: 34110510 Free PMC article. Review.
-
Phase II Multi-institutional Clinical Trial Result of Concurrent Cetuximab and Nivolumab in Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma.Clin Cancer Res. 2022 Jun 1;28(11):2329-2338. doi: 10.1158/1078-0432.CCR-21-3849. Clin Cancer Res. 2022. PMID: 35344035 Free PMC article. Clinical Trial.
-
The Potential of CD16 on Plasma-Derived Exosomes as a Liquid Biomarker in Head and Neck Cancer.Int J Mol Sci. 2020 May 26;21(11):3739. doi: 10.3390/ijms21113739. Int J Mol Sci. 2020. PMID: 32466374 Free PMC article.
-
Perspectives in immunotherapy: meeting report from the Immunotherapy Bridge (29-30 November, 2017, Naples, Italy).J Immunother Cancer. 2018 Jul 11;6(1):69. doi: 10.1186/s40425-018-0377-z. J Immunother Cancer. 2018. PMID: 29996914 Free PMC article. Review.
References
-
- Chow LQ, Morishima C, Eaton KD, Baik CS, Goulart BH, Anderson LN, et al. Phase 1b trial of the toll-like receptor 8 agonist, motolimod (VTX-2337), combined with cetuximab in patients with recurrent or metastatic SCCHN. Clin Cancer Res 2017;23:2442–50. - PubMed
-
- Trotta AM, Ottaiano A, Romano C, Nasti G, Nappi A, De Divitiis C, et al. Prospective evaluation of cetuximab-mediated antibody-dependent cell cytotoxicity in metastatic colorectal cancer patients predicts treatment efficacy. Cancer Immunol Res 2016;4:366–74. - PubMed
-
- Pahl JH, Ruslan SE, Buddingh EP, Santos SJ, Szuhai K, Serra M, et al. Anti-EGFR antibody cetuximab enhances the cytolytic activity of natural killer cells toward osteosarcoma. Clin Cancer Res 2012;18:432–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

