Growth of Caenorhabditis elegans in Defined Media Is Dependent on Presence of Particulate Matter
- PMID: 29223977
- PMCID: PMC5919726
- DOI: 10.1534/g3.117.300325
Growth of Caenorhabditis elegans in Defined Media Is Dependent on Presence of Particulate Matter
Abstract
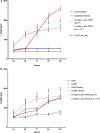
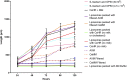
Caenorhabditis elegans are typically cultured in a monoxenic medium consisting of live bacteria. However, this introduces a secondary organism to experiments, and restricts the manipulation of the nutritional environment. Due to the intricate link between genes and environment, greater control and understanding of nutritional factors are required to push the C. elegans field into new areas. For decades, attempts to develop a chemically defined, axenic medium as an alternative for culturing C. elegans have been made. However, the mechanism by which the filter feeder C. elegans obtains nutrients from these liquid media is not known. Using a fluorescence-activated cell sorting based approach, we demonstrate growth in all past axenic C. elegans media to be dependent on the presence of previously unknown particles. This particle requirement of C. elegans led to development of liposome-based, nanoparticle culturing that allows full control of nutrients delivered to C. elegans.
Keywords: Caenorhabditis elegans; axenic media; feeding; liposomes; nutrition.
Copyright © 2018 Flavel et al.
Figures


References
-
- Avery L., 1993. Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J. Exp. Biol. 175: 283–297. - PubMed
-
- Avery, L., and Y. J. You, 2012 C. elegans feeding (May 21, 2012). WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.150.1, http://www.wormbook.org.
-
- Brans G., Schroën C. G. P. H., Van der Sman R. G. M., Boom R. M., 2004. Membrane fractionation of milk: state of the art and challenges. J. Membr. Sci. 243: 263–272.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
