Sex Differences in Androgen Regulation of Metabolism in Nonhuman Primates
- PMID: 29224110
- PMCID: PMC5893331
- DOI: 10.1007/978-3-319-70178-3_24
Sex Differences in Androgen Regulation of Metabolism in Nonhuman Primates
Abstract
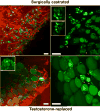
The in-depth characterization of sex differences relevant to human physiology requires the judicious use of a variety of animal models and human clinical data. Nonhuman primates (NHPs) represent an important experimental system that bridges rodent studies and clinical investigations. NHP studies have been especially useful in understanding the role of sex hormones in development and metabolism and also allow the elucidation of the effects of pertinent dietary influences on physiology pertinent to disease states such as obesity and diabetes. This chapter summarizes the current state of our understanding of androgen effects on male and female NHP metabolism relevant to hypogonadism in human males and polycystic ovary syndrome in human females. This review will also focus on the interaction between altered androgen levels and dietary restriction and excess, in particular the Western-style diet that underlies significant human pathophysiology.
Figures
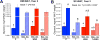


Similar articles
-
The Role of Androgen Excess in Metabolic Dysfunction in Women : Androgen Excess and Female Metabolic Dysfunction.Adv Exp Med Biol. 2017;1043:597-608. doi: 10.1007/978-3-319-70178-3_26. Adv Exp Med Biol. 2017. PMID: 29224112 Review.
-
[The pathomechanism of polycystic ovary syndrome in the light of recent research findings].Orv Hetil. 2012 Oct 7;153(40):1567-9. doi: 10.1556/OH.2012.29467. Orv Hetil. 2012. PMID: 23022879 Review. Hungarian.
-
The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency.Hum Reprod. 2014 Oct 10;29(10):2083-91. doi: 10.1093/humrep/deu198. Epub 2014 Aug 7. Hum Reprod. 2014. PMID: 25104855 Review.
-
Western-style diet, sex steroids and metabolism.Biochim Biophys Acta Mol Basis Dis. 2017 May;1863(5):1147-1155. doi: 10.1016/j.bbadis.2016.05.025. Epub 2016 Jun 3. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27264336 Review.
-
Bromocriptine, sex steroid metabolism, and menstrual patterns in the polycystic ovary syndrome.Ann Intern Med. 1986 Jul;105(1):129-30. doi: 10.7326/0003-4819-105-1-129. Ann Intern Med. 1986. PMID: 3717784 No abstract available.
Cited by
-
An Evolutionary Model for the Ancient Origins of Polycystic Ovary Syndrome.J Clin Med. 2023 Sep 22;12(19):6120. doi: 10.3390/jcm12196120. J Clin Med. 2023. PMID: 37834765 Free PMC article.
-
Diet-Induced Obesity Elicits Macrophage Infiltration and Reduction in Spine Density in the Hypothalami of Male but Not Female Mice.Front Immunol. 2018 Sep 11;9:1992. doi: 10.3389/fimmu.2018.01992. eCollection 2018. Front Immunol. 2018. PMID: 30254630 Free PMC article.
-
Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome.Endocr Rev. 2020 Jul 1;41(4):bnaa010. doi: 10.1210/endrev/bnaa010. Endocr Rev. 2020. PMID: 32310267 Free PMC article. Review.
-
POLYCYSTIC OVARY SYNDROME: ORIGINS AND IMPLICATIONS: Polycystic ovary syndrome: an evolutionary metabolic adaptation.Reproduction. 2025 Mar 24;169(4):e250021. doi: 10.1530/REP-25-0021. Print 2025 Apr 1. Reproduction. 2025. PMID: 40080428 Review.
-
Naturally Occurring and Experimentally Induced Rhesus Macaque Models for Polycystic Ovary Syndrome: Translational Gateways to Clinical Application.Med Sci (Basel). 2019 Nov 27;7(12):107. doi: 10.3390/medsci7120107. Med Sci (Basel). 2019. PMID: 31783681 Free PMC article. Review.
References
-
- Karp NA, Mason J, Beaudet AL, Benjamini Y, Bower L, Braun RE, Brown SDM, Chesler EJ, Dickinson ME, Flenniken AM, Fuchs H, Angelis MH, Gao X, Guo S, Greenaway S, Heller R, Herault Y, Justice MJ, Kurbatova N, Lelliott CJ, Lloyd KCK, Mallon AM, Mank JE, Masuya H, McKerlie C, Meehan TF, Mott RF, Murray SA, Parkinson H, Ramirez-Solis R, Santos L, Seavitt JR, Smedley D, Sorg T, Speak AO, Steel KP, Svenson KL, Wakana S, West D, Wells S, Westerberg H, Yaacoby S, White JK. Prevalence of sexual dimorphism in mammalian phenotypic traits. Nature communications. 2017;8:15475. doi: 10.1038/ncomms15475. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

