Circadian peak dopaminergic activity response at the biological clock pacemaker (suprachiasmatic nucleus) area mediates the metabolic responsiveness to a high-fat diet
- PMID: 29224246
- PMCID: PMC5817247
- DOI: 10.1111/jne.12563
Circadian peak dopaminergic activity response at the biological clock pacemaker (suprachiasmatic nucleus) area mediates the metabolic responsiveness to a high-fat diet
Abstract
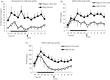
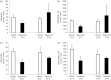
Among vertebrate species of the major vertebrate classes in the wild, a seasonal rhythm of whole body fuel metabolism, oscillating from a lean to obese condition, is a common biological phenomenon. This annual cycle is driven in part by annual changes in the circadian dopaminergic signalling at the suprachiasmatic nuclei (SCN), with diminution of circadian peak dopaminergic activity at the SCN facilitating development of the seasonal obese insulin-resistant condition. The present study investigated whether such an ancient circadian dopamine-SCN activity system for expression of the seasonal obese, insulin-resistant phenotype may be operative in animals made obese amd insulin resistant by high-fat feeding and, if so, whether reinstatement of the circadian dopaminergic peak at the SCN would be sufficient to reverse the adverse metabolic impact of the high-fat diet without any alteration of caloric intake. First, we identified the supramammillary nucleus as a novel site providing the majority of dopaminergic neuronal input to the SCN. We further identified dopamine D2 receptors within the peri-SCN region as being functional in mediating SCN responsiveness to local dopamine. In lean, insulin-sensitive rats, the peak in the circadian rhythm of dopamine release at the peri-SCN coincided with the daily peak in SCN electrophysiological responsiveness to local dopamine administration. However, in rats made obese and insulin resistant by high-fat diet (HFD) feeding, these coincident circadian peak activities were both markedly attenuated or abolished. Reinstatement of the circadian peak in dopamine level at the peri-SCN by its appropriate circadian-timed daily microinjection to this area (but not outside this circadian time-interval) abrogated the obese, insulin-resistant condition without altering the consumption of the HFD. These findings suggest that the circadian peak of dopaminergic activity at the peri-SCN/SCN is a key modulator of metabolism and the responsiveness to adverse metabolic consequences of HFD consumption.
Keywords: circadian; diabetes; dopamine; insulin sensitivity; suprachiasmatic nuclei.
© 2017 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Figures





Similar articles
-
Time-of-Day-Dependent Effects of Bromocriptine to Ameliorate Vascular Pathology and Metabolic Syndrome in SHR Rats Held on High Fat Diet.Int J Mol Sci. 2021 Jun 7;22(11):6142. doi: 10.3390/ijms22116142. Int J Mol Sci. 2021. PMID: 34200262 Free PMC article.
-
Association of the antidiabetic effects of bromocriptine with a shift in the daily rhythm of monoamine metabolism within the suprachiasmatic nuclei of the Syrian hamster.Chronobiol Int. 2000 Mar;17(2):155-72. doi: 10.1081/cbi-100101040. Chronobiol Int. 2000. PMID: 10757461
-
Experimental dopaminergic neuron lesion at the area of the biological clock pacemaker, suprachiasmatic nuclei (SCN) induces metabolic syndrome in rats.Diabetol Metab Syndr. 2021 Jan 23;13(1):11. doi: 10.1186/s13098-021-00630-x. Diabetol Metab Syndr. 2021. PMID: 33485386 Free PMC article.
-
Brain Dopamine-Clock Interactions Regulate Cardiometabolic Physiology: Mechanisms of the Observed Cardioprotective Effects of Circadian-Timed Bromocriptine-QR Therapy in Type 2 Diabetes Subjects.Int J Mol Sci. 2023 Aug 26;24(17):13255. doi: 10.3390/ijms241713255. Int J Mol Sci. 2023. PMID: 37686060 Free PMC article. Review.
-
Dopamine Signaling in Circadian Photoentrainment: Consequences of Desynchrony.Yale J Biol Med. 2019 Jun 27;92(2):271-281. eCollection 2019 Jun. Yale J Biol Med. 2019. PMID: 31249488 Free PMC article. Review.
Cited by
-
Genetically encoded fluorescent sensors for imaging neuronal dynamics in vivo.J Neurochem. 2023 Feb;164(3):284-308. doi: 10.1111/jnc.15608. Epub 2022 Apr 9. J Neurochem. 2023. PMID: 35285522 Free PMC article. Review.
-
Bromocriptine-QR Therapy Reduces Sympathetic Tone and Ameliorates a Pro-Oxidative/Pro-Inflammatory Phenotype in Peripheral Blood Mononuclear Cells and Plasma of Type 2 Diabetes Subjects.Int J Mol Sci. 2022 Aug 9;23(16):8851. doi: 10.3390/ijms23168851. Int J Mol Sci. 2022. PMID: 36012132 Free PMC article.
-
Circadian Dysfunction in Adipose Tissue: Chronotherapy in Metabolic Diseases.Biology (Basel). 2023 Aug 2;12(8):1077. doi: 10.3390/biology12081077. Biology (Basel). 2023. PMID: 37626963 Free PMC article. Review.
-
Bromocriptine mesylate improves glucose tolerance and disposal in a high-fat-fed canine model.Am J Physiol Endocrinol Metab. 2020 Jul 1;319(1):E133-E145. doi: 10.1152/ajpendo.00479.2019. Epub 2020 May 27. Am J Physiol Endocrinol Metab. 2020. PMID: 32459527 Free PMC article.
-
Time-of-Day-Dependent Effects of Bromocriptine to Ameliorate Vascular Pathology and Metabolic Syndrome in SHR Rats Held on High Fat Diet.Int J Mol Sci. 2021 Jun 7;22(11):6142. doi: 10.3390/ijms22116142. Int J Mol Sci. 2021. PMID: 34200262 Free PMC article.
References
-
- Meier AH, Cincotta AH. Circadian rhythms regulate the expression of the thrifty phenotype/phenotype. Diabetes Rev. 1996;4:464‐487. Full text is available at www.veroscience.com/pubs.html
-
- Cincotta AH. Hypothalamic role in the insulin resistance syndrome In: Hansen B, Shaffrir E, eds. Insulin Resistance and Insulin Resistance Syndrome. London: Taylor and Francis; 2002:271‐312.
-
- de Souza CJ, Meier AH. Circadian and seasonal variations of plasma insulin and cortisol concentrations in the Syrian hamster, Mesocricetus auratus . Chronobiol Int. 1987;4:141‐151. - PubMed
-
- Cincotta AH, Schiller BC, Meier AH. Bromocriptine inhibits the seasonally occurring obesity, hyperinsulinemia, insulin resistance, and impaired glucose tolerance in the Syrian hamster, Mesocricetus auratus . Metabolism. 1991;40:639‐644. - PubMed
-
- Luo S, Luo J, Cincotta AH. Suprachiasmatic nuclei monoamine metabolism of glucose tolerant versus intolerant hamsters. NeuroReport. 1999;10:2073‐2077. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

