Mechanism of the Ullmann Biaryl Ether Synthesis Catalyzed by Complexes of Anionic Ligands: Evidence for the Reaction of Iodoarenes with Ligated Anionic CuI Intermediates
- PMID: 29224350
- PMCID: PMC5810543
- DOI: 10.1021/jacs.7b11853
Mechanism of the Ullmann Biaryl Ether Synthesis Catalyzed by Complexes of Anionic Ligands: Evidence for the Reaction of Iodoarenes with Ligated Anionic CuI Intermediates
Abstract
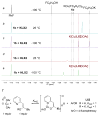
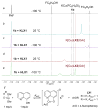
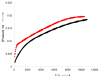
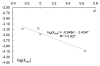
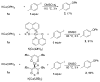
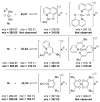
A series of experimental studies, along with DFT calculations, are reported that provide a detailed view into the mechanism of Ullmann coupling of phenols with aryl halides in the presence of catalysts generated from Cu(I) and bidentate, anionic ligands. These studies encompass catalysts containing anionic ligands formed by deprotonation of 8-hydroxyquinoline, 2-pyridylmethyl tert-butyl ketone, and 2,2,6,6-tetramethylheptane-3,5-dione. Three-coordinate, heteroleptic species [Cu(LX)OAr]- were shown by experiment and DFT calculations to be the most stable complexes in catalytic systems containing 8-hydroxyquinoline or 2-pyridylmethyl tert-butyl ketone and to be generated reversibly in the system containing 2,2,6,6-tetramethylheptane-3,5-dione. These heteroleptic complexes were characterized by a combination of 19F NMR, 1H NMR, and UV-vis spectroscopy, as well as ESI-MS. The heteroleptic complexes generated in situ react with iodoarenes to form biaryl ethers in high yields without evidence for an aryl radical intermediate. Measurements of 13C/12C isotope effects showed that oxidative addition of the iodoarene occurs irreversibly. This information, in combination with the kinetic data, shows that oxidative addition occurs to the [Cu(LX)OAr]- complexes and is turnover-limiting. A Hammett analysis of the effect of phenoxide electronic properties on the rate of the reaction of [Cu(LX)OAr]- with iodotoluene also is consistent with oxidative addition of the iodoarene to an anionic phenoxide complex. Calculations by DFT suggest that this oxidative addition is followed by dissociation of I- and reductive elimination of the biaryl ether from the resulting neutral Cu(III) complex.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



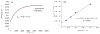
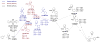








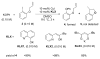
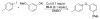
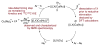
References
-
- Frlan R, Kikelj D. Synthesis. 2006;2006:2271–2285.
-
- Ullmann F, Sponagel P. Ber Dtsch Chem Ges. 1905;38:2211–2212.
-
- Kiyomori A, Marcoux JF, Buchwald SL. Tetrahedron Lett. 1999;40:2657–2660.
- Goodbrand HB, Hu NX. J Org Chem. 1999;64:670–674.
- Gujadhur RK, Bates CG, Venkataraman D. Org Lett. 2001;3:4315–4317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

