Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin's Lymphoma
- PMID: 29224502
- PMCID: PMC5819601
- DOI: 10.1056/NEJMoa1708984
Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin's Lymphoma
Erratum in
-
Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin's Lymphoma.N Engl J Med. 2018 Mar 1;378(9):878. doi: 10.1056/NEJMx180007. N Engl J Med. 2018. PMID: 29490175 No abstract available.
Abstract
Background: Brentuximab vedotin is an anti-CD30 antibody-drug conjugate that has been approved for relapsed and refractory Hodgkin's lymphoma.
Methods: We conducted an open-label, multicenter, randomized phase 3 trial involving patients with previously untreated stage III or IV classic Hodgkin's lymphoma, in which 664 were assigned to receive brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) and 670 were assigned to receive doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD). The primary end point was modified progression-free survival (the time to progression, death, or noncomplete response and use of subsequent anticancer therapy) as adjudicated by an independent review committee. The key secondary end point was overall survival.
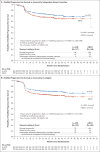
Results: At a median follow-up of 24.6 months, 2-year modified progression-free survival rates in the A+AVD and ABVD groups were 82.1% (95% confidence interval [CI], 78.8 to 85.0) and 77.2% (95% CI, 73.7 to 80.4), respectively, a difference of 4.9 percentage points (hazard ratio for an event of progression, death, or modified progression, 0.77; 95% CI, 0.60 to 0.98; P=0.04). There were 28 deaths with A+AVD and 39 with ABVD (hazard ratio for interim overall survival, 0.73 [95% CI, 0.45 to 1.18]; P=0.20) [corrected]. All secondary efficacy end points trended in favor of A+AVD. Neutropenia occurred in 58% of the patients receiving A+AVD and in 45% of those receiving ABVD; in the A+AVD group, the rate of febrile neutropenia was lower among the 83 patients who received primary prophylaxis with granulocyte colony-stimulating factor than among those who did not (11% vs. 21%). Peripheral neuropathy occurred in 67% of patients in the A+AVD group and in 43% of patients in the ABVD group; 67% of patients in the A+AVD group who had peripheral neuropathy had resolution or improvement at the last follow-up visit. Pulmonary toxicity of grade 3 or higher was reported in less than 1% of patients receiving A+AVD and in 3% of those receiving ABVD. Among the deaths that occurred during treatment, 7 of 9 in the A+AVD group were associated with neutropenia and 11 of 13 in the ABVD group were associated with pulmonary-related toxicity.
Conclusions: A+AVD had superior efficacy to ABVD in the treatment of patients with advanced-stage Hodgkin's lymphoma, with a 4.9 percentage-point lower combined risk of progression, death, or noncomplete response and use of subsequent anticancer therapy at 2 years. (Funded by Millennium Pharmaceuticals and Seattle Genetics; ECHELON-1 ClinicalTrials.gov number, NCT01712490 ; EudraCT number, 2011-005450-60 .).
Figures

Comment in
-
Progress in the Treatment of Hodgkin's Lymphoma.N Engl J Med. 2018 Jan 25;378(4):392-394. doi: 10.1056/NEJMe1715141. Epub 2017 Dec 10. N Engl J Med. 2018. PMID: 29224505 No abstract available.
-
Haematological cancer: Brentuximab effective in untreated Hodgkin lymphoma.Nat Rev Clin Oncol. 2018 Feb;15(2):68. doi: 10.1038/nrclinonc.2017.209. Epub 2017 Dec 28. Nat Rev Clin Oncol. 2018. PMID: 29283169 No abstract available.
-
[Vaccines: first and foremost, a matter of public health].Epidemiol Prev. 2018 Jan-Feb;42(1):3-4. doi: 10.19191/EP18.1.P003.002. Epidemiol Prev. 2018. PMID: 29506348 Italian. No abstract available.
-
Brentuximab Vedotin for Stage III or IV Hodgkin’s Lymphoma.N Engl J Med. 2018 Apr 19;378(16):1558-9. doi: 10.1056/NEJMc1802363. N Engl J Med. 2018. PMID: 29671464 No abstract available.
-
Brentuximab Vedotin for Stage III or IV Hodgkin’s Lymphoma.N Engl J Med. 2018 Apr 19;378(16):1559. doi: 10.1056/NEJMc1802363. N Engl J Med. 2018. PMID: 29671465 No abstract available.
-
Brentuximab Vedotin for Stage III or IV Hodgkin’s Lymphoma.N Engl J Med. 2018 Apr 19;378(16):1559-60. doi: 10.1056/NEJMc1802363. N Engl J Med. 2018. PMID: 29671466 No abstract available.
-
Brentuximab Vedotin for Stage III or IV Hodgkin’s Lymphoma.N Engl J Med. 2018 Apr 19;378(16):1560. doi: 10.1056/NEJMc1802363. N Engl J Med. 2018. PMID: 29671468 No abstract available.
-
Benefit of brentuximab over bleomycin in first-line treatment of advanced-stage Hodgkin lymphoma has not been proven.Blood. 2018 Jul 19;132(3):339-340. doi: 10.1182/blood-2018-04-845438. Epub 2018 Jun 1. Blood. 2018. PMID: 29858234 No abstract available.
-
Brentuximab vedotin for frontline Hodgkin lymphoma: How much will a successful trial cost patients and payers?Eur J Cancer. 2018 Nov;104:252-253. doi: 10.1016/j.ejca.2018.09.021. Epub 2018 Oct 17. Eur J Cancer. 2018. PMID: 30342911 No abstract available.
References
-
- Engert A. ABVD or BEACOPP for advanced Hodgkin lymphoma. J Clin Oncol. 2016;34:1167–9. - PubMed
-
- Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478–84. - PubMed
-
- Carde P, Karrasch M, Fortpied C, et al. Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, international prognostic score ≥ 3, high-risk Hodgkin lymphoma: first results of the phase III EORTC 20012 Intergroup Trial. J Clin Oncol. 2016;34:2028–36. - PubMed
-
- Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31:684–91. - PMC - PubMed
-
- Canellos GP, Duggan D, Johnson J, Niedzwiecki D. How important is bleomycin in the adriamycin + bleomycin + vinblastine + dacarbazine regimen? J Clin Oncol. 2004;22:1532–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
