Injecting Instructions into Premotor Cortex
- PMID: 29224724
- PMCID: PMC5739962
- DOI: 10.1016/j.neuron.2017.11.006
Injecting Instructions into Premotor Cortex
Abstract
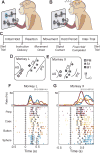
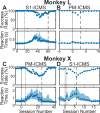
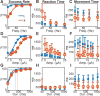
The premotor cortex (PM) receives inputs from parietal cortical areas representing processed visuospatial information, translates that information into programs for particular movements, and communicates those programs to the primary motor cortex (M1) for execution. Consistent with this general function, intracortical microstimulation (ICMS) in the PM of sufficient frequency, amplitude, and duration has been shown to evoke complex movements of the arm and hand that vary systematically depending on the locus of stimulation. Using frequencies and amplitudes too low to evoke muscle activity, however, we found that ICMS in the PM can provide instructions to perform specific reach, grasp, and manipulate movements. These instructed actions were not fixed but rather were learned through associations between the arbitrary stimulation locations and particular movements. Low-amplitude ICMS at different PM locations thus evokes distinguishable experiences that can become associated with specific movements arbitrarily, providing a novel means of injecting information into the nervous system.
Keywords: conditional association; grasping; intracortical microstimulation; learning; manipulation; premotor cortex; primary motor cortex; primary somatosensory cortex; reaching.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Motor systems: Cortical instructions.Nat Rev Neurosci. 2017 Dec 14;19(1):6-7. doi: 10.1038/nrn.2017.164. Nat Rev Neurosci. 2017. PMID: 29238083 No abstract available.
-
Commentary: Injecting Instructions into Premotor Cortex.Front Cell Neurosci. 2018 Mar 27;12:65. doi: 10.3389/fncel.2018.00065. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29637931 Free PMC article. No abstract available.
References
-
- Andersen P, Hagan PJ, Phillips CG, Powell TP. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon’s hand. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1975;188:31–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

