YY1 Is a Structural Regulator of Enhancer-Promoter Loops
- PMID: 29224777
- PMCID: PMC5785279
- DOI: 10.1016/j.cell.2017.11.008
YY1 Is a Structural Regulator of Enhancer-Promoter Loops
Abstract
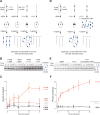
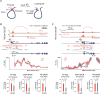
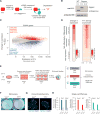
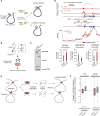
There is considerable evidence that chromosome structure plays important roles in gene control, but we have limited understanding of the proteins that contribute to structural interactions between gene promoters and their enhancer elements. Large DNA loops that encompass genes and their regulatory elements depend on CTCF-CTCF interactions, but most enhancer-promoter interactions do not employ this structural protein. Here, we show that the ubiquitously expressed transcription factor Yin Yang 1 (YY1) contributes to enhancer-promoter structural interactions in a manner analogous to DNA interactions mediated by CTCF. YY1 binds to active enhancers and promoter-proximal elements and forms dimers that facilitate the interaction of these DNA elements. Deletion of YY1 binding sites or depletion of YY1 protein disrupts enhancer-promoter looping and gene expression. We propose that YY1-mediated enhancer-promoter interactions are a general feature of mammalian gene control.
Keywords: chromosome structure; enhancers; gene regulation; promoters.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Comment in
-
YY1 Facilitates Enhancer-Promoter Contacts to Promote Gene Expression.Cancer Discov. 2018 Feb;8(2):135. doi: 10.1158/2159-8290.CD-RW2017-233. Epub 2017 Dec 15. Cancer Discov. 2018. PMID: 29247020
-
Gene expression: The yin and yang of enhancer-promoter interactions.Nat Rev Mol Cell Biol. 2018 Feb;19(2):75. doi: 10.1038/nrm.2017.136. Epub 2017 Dec 20. Nat Rev Mol Cell Biol. 2018. PMID: 29259336 No abstract available.
References
-
- Adhya S. Multipartite genetic control elements: Communication by DNA loop. Annu Rev Genet. 1989;23:227–250. - PubMed
-
- Amoutzias GD, Robertson DL, Van de Peer Y, Oliver SG. Choose your partners: Dimerization in eukaryotic transcription factors. Trends Biochem Sci. 2008;33:220–229. - PubMed
-
- Baniahmad A, Steiner C, Köhne AC, Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990;61:505–514. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

