Tregs: Where We Are and What Comes Next?
- PMID: 29225597
- PMCID: PMC5705554
- DOI: 10.3389/fimmu.2017.01578
Tregs: Where We Are and What Comes Next?
Abstract
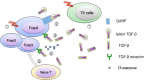
Regulatory T cells are usually recognized as a specialized subset of CD4+ T cells functioning in establishment and maintenance of immune tolerance. Meanwhile, there is emerging evidence that regulatory T cells (Tregs) are also present in various non-lymphoid tissues, and that they have unique phenotypes credited with activities distinct from regulatory function. Their development and function have been described in plenty of manuscripts in the past two decades. However, with the deepening of research in recent years, emerging evidence revealed some novel mechanisms about how Tregs exert their activities. First, we discuss the expanding family of regulatory lymphocytes briefly and then, try to interpret how fork-head box P3 (Foxp3), a master regulator of the regulatory pathway in the development and function of regulatory T cells, functions. Subsequently, another part of our focus is varieties of tissue Tregs. Next, we primarily discuss recent research on how Tregs work and their faceted functions in terms of soluble mediators, functional proteins, and inhibitory receptors. In particular, unless otherwise noted, the term "Treg" is used here to refer specially to the "CD4+CD25+Foxp3+" regulatory cells.
Keywords: Foxp3; amphiregulin; d-mannose; neuropilin-1; regulatory T cells; regulatory innate lymphoid cells.
Figures



References
-
- Ali N, Zirak B, Rodriguez RS, Cotsarelis G, Abbas AK, Rosenblum MD, et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation article regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell (2017) 169(6):1119–29.e11.10.1016/j.cell.2017.05.002 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

