Cellular Senescence in Age-Related Macular Degeneration: Can Autophagy and DNA Damage Response Play a Role?
- PMID: 29225722
- PMCID: PMC5687149
- DOI: 10.1155/2017/5293258
Cellular Senescence in Age-Related Macular Degeneration: Can Autophagy and DNA Damage Response Play a Role?
Abstract
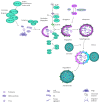
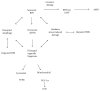
Age-related macular degeneration (AMD) is the main reason of blindness in developed countries. Aging is the main AMD risk factor. Oxidative stress, inflammation and some genetic factors play a role in AMD pathogenesis. AMD is associated with the degradation of retinal pigment epithelium (RPE) cells, photoreceptors, and choriocapillaris. Lost RPE cells in the central retina can be replaced by their peripheral counterparts. However, if they are senescent, degenerated regions in the macula cannot be regenerated. Oxidative stress, a main factor of AMD pathogenesis, can induce DNA damage response (DDR), autophagy, and cell senescence. Moreover, cell senescence is involved in the pathogenesis of many age-related diseases. Cell senescence is the state of permanent cellular division arrest and concerns only mitotic cells. RPE cells, although quiescent in the retina, can proliferate in vitro. They can also undergo oxidative stress-induced senescence. Therefore, cellular senescence can be considered as an important molecular pathway of AMD pathology, resulting in an inability of the macula to regenerate after degeneration of RPE cells caused by a factor inducing DDR and autophagy. It is too early to speculate about the role of the mutual interplay between cell senescence, autophagy, and DDR, but this subject is worth further studies.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

