Sex determination mode does not affect body or genital development of the central bearded dragon (Pogona vitticeps)
- PMID: 29225770
- PMCID: PMC5716226
- DOI: 10.1186/s13227-017-0087-5
Sex determination mode does not affect body or genital development of the central bearded dragon (Pogona vitticeps)
Abstract
Background: The development of male- or female-specific phenotypes in squamates is typically controlled by either temperature-dependent sex determination (TSD) or chromosome-based genetic sex determination (GSD). However, while sex determination is a major switch in individual phenotypic development, it is unknownhow evolutionary transitions between GSD and TSD might impact on the evolution of squamate phenotypes, particularly the fast-evolving and diverse genitalia. Here, we take the unique opportunity of studying the impact of both sex determination mechanisms on the embryological development of the central bearded dragon (Pogona vitticeps). This is possible because of the transitional sex determination system of this species, in which genetically male individuals reverse sex at high incubation temperatures. This can trigger the evolutionary transition of GSD to TSD in a single generation, making P. vitticeps an ideal model organism for comparing the effects of both sex determination processes in the same species.
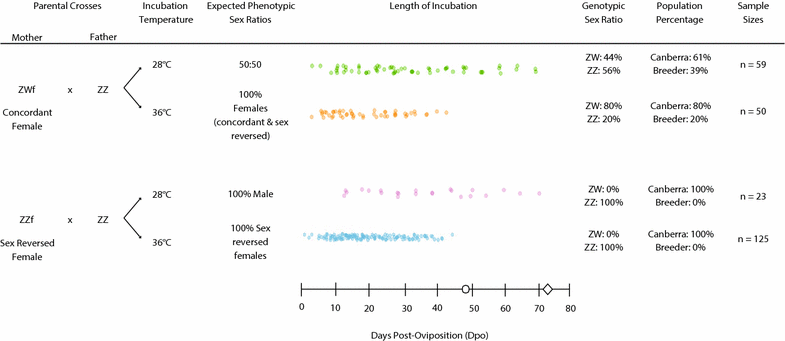
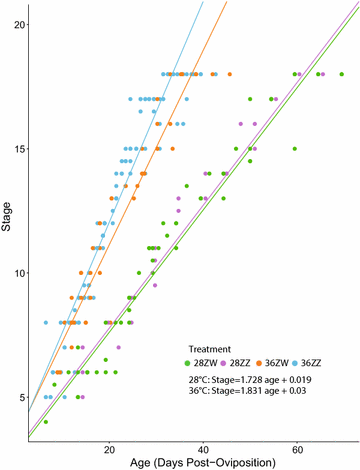
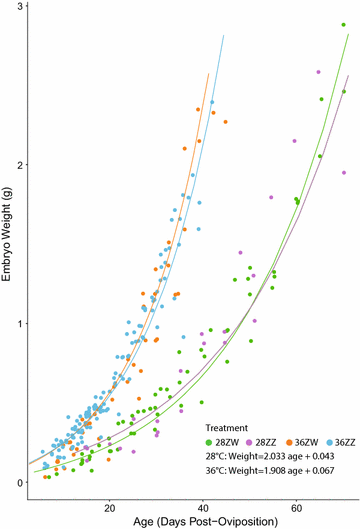
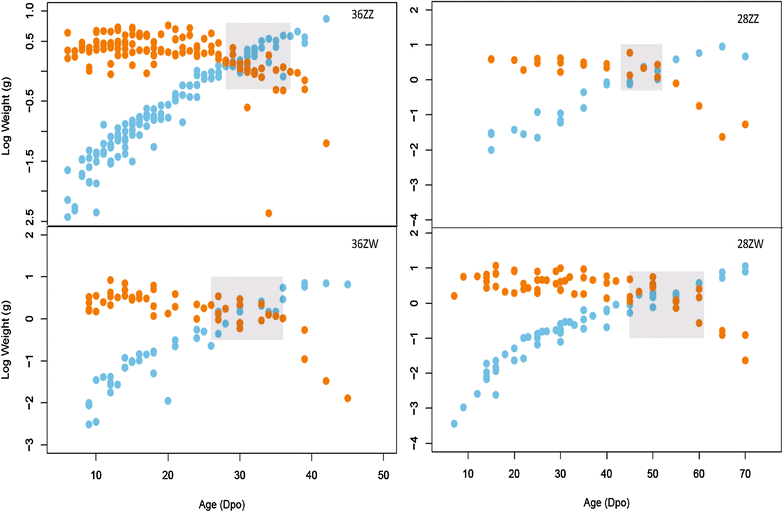
Results: We conducted four incubation experiments on 265 P. vitticeps eggs, covering two temperature regimes ("normal" at 28 °C and "sex reversing" at 36 °C) and the two maternal sexual genotypes (concordant ZW females or sex-reversed ZZ females). From this, we provide the first detailed staging system for the species, with a focus on genital and limb development. This was augmented by a new sex chromosome identification methodology for P. vitticeps that is non-destructive to the embryo. We found a strong correlation between embryo age and embryo stage. Aside from faster growth in 36 °C treatments, body and external genital development was entirely unperturbed by temperature, sex reversal or maternal sexual genotype. Unexpectedly, all females developed hemipenes (the genital phenotype of adult male P. vitticeps), which regress close to hatching.
Conclusions: The tight correlation between embryo age and embryo stage allows the precise targeting of specific developmental periods in the emerging field of molecular research on P. vitticeps. The stability of genital development in all treatments suggests that the two sex-determining mechanisms have little impact on genital evolution, despite their known role in triggering genital development. Hemipenis retention in developing female P. vitticeps, together with frequent occurrences of hemipenis-like structures during development in other squamate species, raises the possibility of a bias towards hemipenis formation in the ancestral developmental programme for squamate genitalia.
Keywords: Embryonic development; Genitalia; Sex reversal; Squamates; Staging table.
Figures
Similar articles
-
ZW Sex Chromosomes in Australian Dragon Lizards (Agamidae) Originated from a Combination of Duplication and Translocation in the Nucleolar Organising Region.Genes (Basel). 2019 Oct 30;10(11):861. doi: 10.3390/genes10110861. Genes (Basel). 2019. PMID: 31671601 Free PMC article.
-
Skull Development, Ossification Pattern, and Adult Shape in the Emerging Lizard Model Organism Pogona vitticeps: A Comparative Analysis With Other Squamates.Front Physiol. 2018 Mar 28;9:278. doi: 10.3389/fphys.2018.00278. eCollection 2018. Front Physiol. 2018. PMID: 29643813 Free PMC article.
-
Metabolic consequences of sex reversal in two lizard species: a test of the like-genotype and like-phenotype hypotheses.J Exp Biol. 2023 Jul 1;226(13):jeb245657. doi: 10.1242/jeb.245657. Epub 2023 Jul 14. J Exp Biol. 2023. PMID: 37309620 Free PMC article.
-
Temperature-Induced Sex Reversal in Reptiles: Prevalence, Discovery, and Evolutionary Implications.Sex Dev. 2021;15(1-3):148-156. doi: 10.1159/000515687. Epub 2021 Jun 10. Sex Dev. 2021. PMID: 34111872 Review.
-
Squamates as a model to understand key dental features of vertebrates.Dev Biol. 2024 Dec;516:1-19. doi: 10.1016/j.ydbio.2024.07.011. Epub 2024 Jul 26. Dev Biol. 2024. PMID: 39069116 Review.
Cited by
-
Developmental asynchrony and antagonism of sex determination pathways in a lizard with temperature-induced sex reversal.Sci Rep. 2018 Oct 5;8(1):14892. doi: 10.1038/s41598-018-33170-y. Sci Rep. 2018. PMID: 30291276 Free PMC article.
-
Identification of Y chromosome markers in the eastern three-lined skink (Bassiana duperreyi) using in silico whole genome subtraction.BMC Genomics. 2020 Sep 29;21(1):667. doi: 10.1186/s12864-020-07071-2. BMC Genomics. 2020. PMID: 32993477 Free PMC article.
-
Developmental dynamics of sex reprogramming by high incubation temperatures in a dragon lizard.BMC Genomics. 2022 Apr 22;23(1):322. doi: 10.1186/s12864-022-08544-2. BMC Genomics. 2022. PMID: 35459109 Free PMC article.
-
Ovotestes suggest cryptic genetic influence in a reptile model for temperature-dependent sex determination.Proc Biol Sci. 2021 Jan 27;288(1943):20202819. doi: 10.1098/rspb.2020.2819. Epub 2021 Jan 20. Proc Biol Sci. 2021. PMID: 33467998 Free PMC article.
-
Truncated jarid2 and kdm6b transcripts are associated with temperature-induced sex reversal during development in a dragon lizard.Sci Adv. 2022 Apr 22;8(16):eabk0275. doi: 10.1126/sciadv.abk0275. Epub 2022 Apr 20. Sci Adv. 2022. PMID: 35442724 Free PMC article.
References
-
- Norris D, Lopez K. Hormones and reproduction of vertebrates reptiles. Burlington: Elsevier Science; 2011.
-
- Valenzuela N, Lance V. Temperature-dependent sex determination in vertebrates. Washington: Smithsonian Books; 2004.
LinkOut - more resources
Full Text Sources
Other Literature Sources

