Efficacy of sonographic and biological pleurodesis indicators of malignant pleural effusion (SIMPLE): protocol of a randomised controlled trial
- PMID: 29225889
- PMCID: PMC5708313
- DOI: 10.1136/bmjresp-2017-000225
Efficacy of sonographic and biological pleurodesis indicators of malignant pleural effusion (SIMPLE): protocol of a randomised controlled trial
Abstract
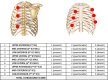
Introduction: Malignant pleural effusion (MPE) is common and currently in UK there are an estimated 50 000 new cases of MPE per year. Talc pleurodesis remains one of the most popular methods for fluid control. The value of thoracic ultrasound (TUS) imaging, before and after pleurodesis, in improving the quality and efficacy of care for patients with MPE remains unknown. Additionally, biomarkers of successful pleurodesis including measurement of pleural fluid proteins have not been validated in prospective studies.The SIMPLE trial is an appropriately powered, multicentre, randomised controlled trial designed to assess 'by the patient bedside' use of TUS imaging and pleural fluid analysis in improving management of MPE.
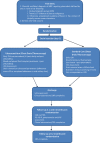
Methods and analysis: 262 participants with a confirmed MPE requiring intervention will be recruited from hospitals in UK and The Netherlands. Participants will be randomised (1:1) to undergo either chest drain insertion followed by instillation of sterile talc, or medical thoracoscopy and simultaneous poudrage. The allocated procedure will be done while the patient is hospitalised, and within 3 days of randomisation. Following hospital discharge, participants will be followed up at 1, 3 and 12 months. The primary outcome measure is the length of hospital stay during initial hospitalisation.
Ethics and dissemination: The trial has received ethical approval from the South Central-Oxford C Research Ethics Committee (Reference number 15/SC/0600). The Trial Steering Committee includes an independent chair and members, and a patient representative. The trial results will be published in a peer-reviewed journal and presented at international conferences.
Trial registration number: ISRCTN: 16441661.
Keywords: mesothelioma; pleural disease.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- American Thoracic S. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987–2001. doi:10.1164/ajrccm.162.5.ats8-00 - DOI - PubMed
-
- Dresler CM. Systemic distribution of talc. Chest 1999;116:266 doi:10.1378/chest.116.1.266 - DOI - PubMed
-
- Cr UK. Incidence of common cancers, 2005.
-
- Roberts ME, Neville E, Berrisford RG, et al. . Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii32–40. doi:10.1136/thx.2010.136994 - DOI - PubMed
-
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974;33:916–22. doi:10.1002/1097-0142(197404)33:4<916::AID-CNCR2820330405>3.0.CO;2-U - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
