Conformal phased surfaces for wireless powering of bioelectronic microdevices
- PMID: 29226018
- PMCID: PMC5722470
- DOI: 10.1038/s41551-017-0043
Conformal phased surfaces for wireless powering of bioelectronic microdevices
Abstract
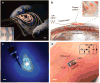
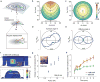
Wireless powering could enable the long-term operation of advanced bioelectronic devices within the human body. Although both enhanced powering depth and device miniaturization can be achieved by shaping the field pattern within the body, existing electromagnetic structures do not provide the spatial phase control required to synthesize such patterns. Here, we describe the design and operation of conformal electromagnetic structures, termed phased surfaces, that interface with non-planar body surfaces and optimally modulate the phase response to enhance the performance of wireless powering. We demonstrate that the phased surfaces can wirelessly transfer energy across anatomically heterogeneous tissues in large animal models, powering miniaturized semiconductor devices (<12 mm3) deep within the body (>4 cm). As an illustration of in vivo operation, we wirelessly regulated cardiac rhythm by powering miniaturized stimulators at multiple endocardial sites in a porcine animal model.
Figures



References
-
- Chandrakasan AP, Verma N, Daly DC. Ultralow-power electronics for biomedical applications. Annu Rev Biomed Eng. 2008;10:247–274. - PubMed
-
- Leonov V. Thermoelectric energy harvesting of human body heat for wearable sensors. IEEE Sensors J. 2013;13:2284–2291.
-
- Schuder J, Stephenson H, Jr, Townsend J. High level electromagnetic energy transfer through a closed chest wall. Inst Radio Engrs Int Conv Record. 1961;9:119–126.
-
- Jow UM, Ghovanloo M. Design and optimization of printed spiral coils for efficient transcutaneous inductive power transmission. IEEE Trans Biomed Circuits Syst. 2008;1:193–202. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

