Leptin-induced migration and angiogenesis in rheumatoid arthritis is mediated by reactive oxygen species
- PMID: 29226077
- PMCID: PMC5715350
- DOI: 10.1002/2211-5463.12326
Leptin-induced migration and angiogenesis in rheumatoid arthritis is mediated by reactive oxygen species
Abstract
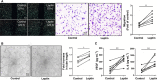
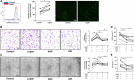
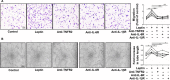
Rheumatoid arthritis (RA) is a progressive autoimmune disease affecting the joints. In this study, we investigated the role of the pro-angiogenic factor leptin in regulating reactive oxygen species (ROS) to promote cell migration and angiogenesis in RA. We showed that leptin triggered RA fibroblast-like synoviocyte (FLS) migration by increased ROS expression. Additionally, leptin enhanced human umbilical vein endothelial cell (HUVEC) tube formation in a ROS/hypoxia-inducible factor-1α-dependent manner, accompanied by increased production of vascular endothelial growth factor and interleukin (IL)-6. We also revealed that antagonists of tumor necrosis factor, IL-6 and IL-1β down-regulated ROS production of RA FLS induced by leptin, which subsequently attenuated RA FLS migration and HUVEC tube formation. These findings demonstrated that leptin might play an important role in RA FLS migration and HUVEC angiogenesis.
Keywords: angiogenesis; leptin; migration; reactive oxygen species; rheumatoid arthritis.
Figures


Similar articles
-
Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models.Int Immunopharmacol. 2009 Mar;9(3):268-76. doi: 10.1016/j.intimp.2008.11.005. Epub 2008 Dec 25. Int Immunopharmacol. 2009. PMID: 19111632
-
TACE-dependent amphiregulin release is induced by IL-1β and promotes cell invasion in fibroblast-like synoviocytes in rheumatoid arthritis.Rheumatology (Oxford). 2014 Feb;53(2):260-9. doi: 10.1093/rheumatology/ket350. Epub 2013 Nov 6. Rheumatology (Oxford). 2014. PMID: 24196392
-
CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients.Arthritis Rheum. 2004 Dec;50(12):3866-77. doi: 10.1002/art.20615. Arthritis Rheum. 2004. PMID: 15593223
-
Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids.Arthritis Rheum. 1999 Aug;42(8):1601-8. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. Arthritis Rheum. 1999. PMID: 10446857
-
Fibroblast-like synoviocytes-dependent effector molecules as a critical mediator for rheumatoid arthritis: Current status and future directions.Int Rev Immunol. 2017 Jan 2;36(1):20-30. doi: 10.1080/08830185.2016.1269175. Epub 2017 Jan 19. Int Rev Immunol. 2017. PMID: 28102734 Review.
Cited by
-
Role of Leptin and Adiponectin in Carcinogenesis.Cancers (Basel). 2023 Aug 24;15(17):4250. doi: 10.3390/cancers15174250. Cancers (Basel). 2023. PMID: 37686525 Free PMC article. Review.
-
Time-restricted feeding alleviates arthritis symptoms augmented by high-fat diet.Front Immunol. 2025 Feb 13;16:1512328. doi: 10.3389/fimmu.2025.1512328. eCollection 2025. Front Immunol. 2025. PMID: 40018036 Free PMC article.
-
A novel 3D spheroid model of rheumatoid arthritis synovial tissue incorporating fibroblasts, endothelial cells, and macrophages.Front Immunol. 2023 Jul 20;14:1188835. doi: 10.3389/fimmu.2023.1188835. eCollection 2023. Front Immunol. 2023. PMID: 37545512 Free PMC article.
-
The effect of nicotinamide adenine dinucleotide phosphate oxidase 4 on migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis.Arthritis Res Ther. 2020 May 15;22(1):116. doi: 10.1186/s13075-020-02204-0. Arthritis Res Ther. 2020. PMID: 32414400 Free PMC article.
-
Atherosclerosis in Rheumatoid Arthritis: Promoters and Opponents.Clin Rev Allergy Immunol. 2020 Feb;58(1):1-14. doi: 10.1007/s12016-018-8714-z. Clin Rev Allergy Immunol. 2020. PMID: 30259381 Review.
References
-
- Eisinger K, Bauer S, Schäffler A, Walter R, Neumann E, Buechler C, Müller‐Ladner U and Frommer KW (2012) Chemerin induces CCL2 and TLR4 in synovial fibroblasts of patients with rheumatoid arthritis and osteoarthritis. Exp Mol Pathol 92, 90–96. - PubMed
-
- Park SY, Lee SW, Kim HY, Lee WS, Hong KW and Kim CD (2015) HMGB1 induces angiogenesis in rheumatoid arthritis via HIF‐1α activation. Eur J Immunol 45, 1216–1227. - PubMed
-
- Westra J, Molema G and Kallenberg CG (2010) Hypoxia‐inducible factor‐1 as regulator of angiogenesis in rheumatoid arthritis‐therapeutic implications. Curr Med Chem 17, 254–263. - PubMed
-
- Sun HL, Liu YN, Huang YT, Pan SL, Huang DY, Guh JH, Lee FY, Kao SC and Teng CM (2007) YC‐1 inhibits HIF‐1 expression in prostate cancer cells: contribution of Akt/NF‐kappaB signaling to HIF‐1alpha accumulation during hypoxia. Oncogene 26, 3941–3951. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

